Anion Analysis Page 4
ADVERTISEMENT
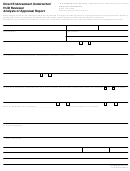
Chemistry 112: Anion Analysis
Page 10
2. Write the balanced, net ionic equation for the reaction occurring when
+
-
(a) Ag
is added to the Cl
containing solution
+
3-
(b) Ag
is added to the PO
containing solution
4
3. When some precipitates dissolve in HCl or HNO
, the reason is that a
3
more “stable” or less strong acid than either HCl or HNO
is formed. For
3
example, barium phosphate, which is normally insoluble in water, dissolves
in HCl because the weaker acid H
PO
(phosphoric acid) is formed.
3
4
(s) + 6 HCl(aq) → 2 H
Ba
(PO
)
PO
(aq) + 3 BaCl
(aq)
3
4
2
3
4
2
Write a balanced equation to show how insoluble silver(I) phosphate,
Ag
PO
, can dissolve in the strong acid HNO
for a similar reason.
3
4
3
Revised: December 2005
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4