Elements And Isotopes Page 2
ADVERTISEMENT
Elements and Isotopes
THINK VISUALLY
2.
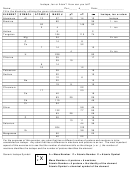
The diagrams show models of carbon isotopes. Complete the
diagrams by drawing in the rest of the atomic particles, remember that the number at the end
represents that isotopes specific mass.
6p
6p
8n
7n
Nonradioactive
Radioactive
carbon-13
carbon-14
Use your completed diagrams to answer Questions 3–4.
3. Identify two differences between carbon-12 and carbon-14.
Mass is different (12 amu vs. 14 amu)
Neutron count is different (7 vs. 8)
4. Identify two ways in which carbon-12, carbon-13, and carbon-14 are alike.
All have the same proton count (6 protons)
All have the same electron count (6 protons)
For Questions 5–7, complete each statement by writing the correct word or words.
Choice of words: ions, isotopes, atoms, electrons
5. A chemical element is a pure substance that consists entirely of one type of atoms.
6. Atoms of the same element that differ in the number of neutrons they contain are called
isotopes.
7. An atom is made up of protons, neutrons, and electrons.
Chemical Bonds
10. Sea salt contains calcium chloride (CaCl
), an ionic compound similar to table salt. One atom
2
of calcium (atomic number 20) bonds to two atoms of chlorine (atomic number 17). Fill in the
number of protons and electrons in each ion.
Chloride ion
Calcium ion
Chloride ion
Protons 17
Protons 20
Protons 17
Electrons 18
Electrons 18
Electrons 18
2
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2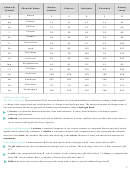 3
3 4
4