Elements And Isotopes Page 4
ADVERTISEMENT
The Water Molecule
For Questions 1–4, write True or False on the line provided.
True 1.
Water is a polar molecule.
False 2.
Hydrogen bonds are an example of
adhesion.
False 4.
A hydrogen bond is stronger than a covalent bond.
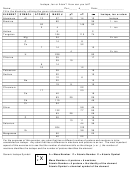
Solutions and Suspensions
5. Complete the table
Substance
Definition
Example(s)
Mixture
Cinnamon sugar
Physical combination of two or more substances
What is being dissolved
Solute
Salt in saltwater
Solvent
What is doing the dissolving
water
Suspension
Mixture of water and nondissolved substance
Blood
A homogeneous mixture where the solvent is mixed
Solution
uniformly throughout.
Acids, Bases, and pH
6. What makes pure water neutral?
Pure water is neutral due to its lack of ions and other dissolved particles. Also it has a pH of 7.
7. What does the pH scale measure?
+
+
The amount of H
ions present. The more acidic it is the more H
ions it has.
8. On the pH scale, indicate which direction is increasingly acidic and which is increasingly basic
Going towards the strong acid is increasingly acidic, and going towards the strong
base is increasingly basic.
–
+
9. Identify two solutions that have more H
ions than OH
ions.
+
-
Anything acid has more H
than OH
. Example: lemon juice and HCl
–
+
10. Identify two solutions that have more OH
ions than H
ions.
Anything basic. Example: NaOH and ammonia
11. How would you buffer a solution that has a pH of 12?
Mix it with a solution that is equally acidic (pH of 2)
Big
Apply the
idea
12. Why are buffers important to living things?
Buffers are important because they help to keep our pH levels in balance. For
example the water in our bodies helps to balance out the acid and bases that we
ingest and create.
4
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4