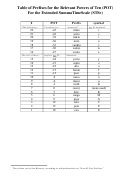
Table Of Standard Reduction Potentials Page 2
ADVERTISEMENT
-
+
-
-
-
ClO
H2O
+ 2 e
→ ClO
+ 2 OH
0.66
2
(aq)
(ℓ)
(aq)
(aq)
3+
-
2+
Fe
+ e
→ Fe
0.771
(aq)
(aq)
2+
-
Hg
+ 2 e
→ 2Hg
0.7973
2
(aq)
(ℓ)
+
-
Ag
+ e
→ Ag
0.7996
(aq)
(s)
-
-
-
-
→ Cl
ClO
+ H
O
+ 2 e
+ 2 OH
0.81
(aq)
2
(ℓ)
(aq)
(aq)
2+
-
Hg
+ 2 e
→ Hg
0.851
(aq)
(ℓ)
2+
-
2+
2 Hg
+ 2 e
→ Hg
0.92
(aq)
2
(aq)
-
+
-
NO
+ 4 H
+ 3 e
→ NO
+ 2 H
O
0.957
3
(aq)
(aq)
(g)
2
(ℓ)
+
-
-
Br
2 e
→ 2 Br
1.066
2 (ℓ)
(aq)
+
-
O
+ 4 H
+ 4 e
→ 2 H
O
1.229
2 (g)
(aq)
2
(ℓ)
2-
+
-
3+
→ 2 Cr
Cr
O
+ 14 H
+ 6 e
+ 7 H
O
1.232
2
7
(aq)
(aq)
(aq)
2
(ℓ)
-
-
Cl
+ 2 e
→ 2Cl
1.35827
2 (g)
(aq)
-
+
+
-
2+
→ Mn
MnO
+ 8 H
5 e
+ 4 H
O
1.507
4
(aq)
(aq)
(aq)
2
(ℓ)
-
+
-
MnO
+ 4 H
+ 3 e
→ MnO
+ 2 H
O
1.679
4
(aq)
(aq)
2 (s)
2
(ℓ)
4+
-
3+
Ce
+ e
→ Ce
1.72
(aq)
(aq)
+
+
-
H
O
+ 2 H
2 e
→ 2 H
O
1.776
2
2 (aq)
(aq)
2
(ℓ)
3+
-
2+
Co
+ e
→ Co
1.92
(aq)
(aq)
2-
-
2-
S
O
+ 2e
→ 2 SO
2.01
2
8
(aq)
4
(aq)
+
-
+
→ O
O
+ 2 H
+ 2 e
H
O
2.076
3 (g)
(aq)
2 (g)
2
(ℓ)
-
-
F
+ 2 e
→ 2 F
2.866
2 (g)
(aq)
20
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2