Standard Reduction Potentials At 25c 298k
ADVERTISEMENT
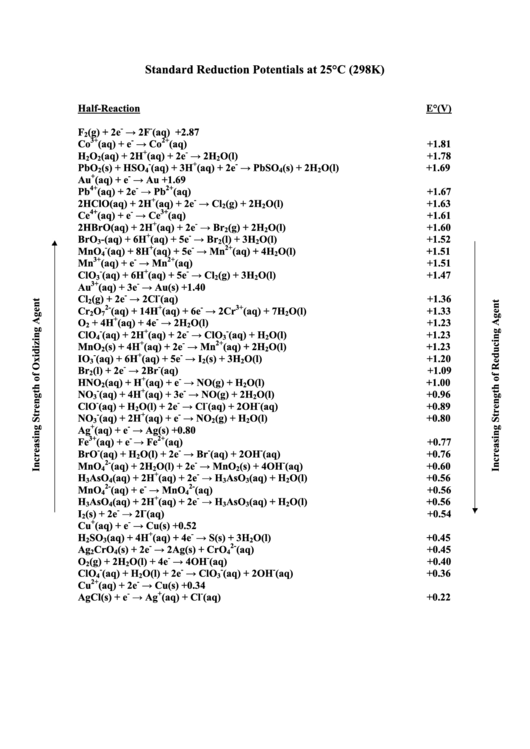
Standard Reduction Potentials at 25°C (298K)
Half-Reaction
E°(V)
-
-
F
(g) + 2e
→ 2F
(aq)
+2.87
2
3+
-
2+
Co
(aq) + e
→ Co
(aq)
+1.81
+
-
H
O
(aq) + 2H
(aq) + 2e
→ 2H
O(l)
+1.78
2
2
2
-
+
-
PbO
(s) + HSO
(aq) + 3H
(aq) + 2e
→ PbSO
(s) + 2H
O(l)
+1.69
2
4
4
2
+
-
Au
(aq) + e
→ Au
+1.69
4+
-
2+
Pb
(aq) + 2e
→ Pb
(aq)
+1.67
+
-
2HClO(aq) + 2H
(aq) + 2e
→ Cl
(g) + 2H
O(l)
+1.63
2
2
4+
-
3+
Ce
(aq) + e
→ Ce
(aq)
+1.61
+
-
2HBrO(aq) + 2H
(aq) + 2e
→ Br
(g) + 2H
O(l)
+1.60
2
2
+
-
BrO
-(aq) + 6H
(aq) + 5e
→ Br
(l) + 3H
O(l)
+1.52
3
2
2
-
+
-
2+
MnO
(aq) + 8H
(aq) + 5e
→ Mn
(aq) + 4H
O(l)
+1.51
4
2
3+
-
2+
Mn
(aq) + e
→ Mn
(aq)
+1.51
-
+
-
ClO
(aq) + 6H
(aq) + 5e
→ Cl
(g) + 3H
O(l)
+1.47
3
2
2
3+
-
Au
(aq) + 3e
→ Au(s)
+1.40
-
-
Cl
(g) + 2e
→ 2Cl
(aq)
+1.36
2
2-
+
-
3+
Cr
O
(aq) + 14H
(aq) + 6e
→ 2Cr
(aq) + 7H
O(l)
+1.33
2
7
2
+
-
O
+ 4H
(aq) + 4e
→ 2H
O(l)
+1.23
2
2
-
+
-
-
ClO
(aq) + 2H
(aq) + 2e
→ ClO
(aq) + H
O(l)
+1.23
4
3
2
+
-
2+
MnO
(s) + 4H
(aq) + 2e
→ Mn
(aq) + 2H
O(l)
+1.23
2
2
-
+
-
IO
(aq) + 6H
(aq) + 5e
→ I
(s) + 3H
O(l)
+1.20
3
2
2
-
-
Br
(l) + 2e
→ 2Br
(aq)
+1.09
2
+
-
HNO
(aq) + H
(aq) + e
→ NO(g) + H
O(l)
+1.00
2
2
-
+
-
NO
(aq) + 4H
(aq) + 3e
→ NO(g) + 2H
O(l)
+0.96
3
2
-
-
-
-
ClO
(aq) + H
O(l) + 2e
→ Cl
(aq) + 2OH
(aq)
+0.89
2
-
+
-
NO
(aq) + 2H
(aq) + e
→ NO
(g) + H
O(l)
+0.80
3
2
2
+
-
Ag
(aq) + e
→ Ag(s)
+0.80
3+
-
2+
Fe
(aq) + e
→ Fe
(aq)
+0.77
-
-
-
-
BrO
(aq) + H
O(l) + 2e
→ Br
(aq) + 2OH
(aq)
+0.76
2
2-
-
-
MnO
(aq) + 2H
O(l) + 2e
→ MnO
(s) + 4OH
(aq)
+0.60
4
2
2
+
-
H
AsO
(aq) + 2H
(aq) + 2e
→ H
AsO
(aq) + H
O(l)
+0.56
3
4
3
3
2
2-
-
2-
MnO
(aq) + e
→ MnO
(aq)
+0.56
4
4
+
-
H
AsO
(aq) + 2H
(aq) + 2e
→ H
AsO
(aq) + H
O(l)
+0.56
3
4
3
3
2
-
-
I
(s) + 2e
→ 2I
(aq)
+0.54
2
+
-
Cu
(aq) + e
→ Cu(s)
+0.52
+
-
H
SO
(aq) + 4H
(aq) + 4e
→ S(s) + 3H
O(l)
+0.45
2
3
2
-
2-
Ag
CrO
(s) + 2e
→ 2Ag(s) + CrO
(aq)
+0.45
2
4
4
-
-
O
(g) + 2H
O(l) + 4e
→ 4OH
(aq)
+0.40
2
2
-
-
-
-
ClO
(aq) + H
O(l) + 2e
→ ClO
(aq) + 2OH
(aq)
+0.36
4
2
3
2+
-
Cu
(aq) + 2e
→ Cu(s)
+0.34
-
+
-
AgCl(s) + e
→ Ag
(aq) + Cl
(aq)
+0.22
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2








