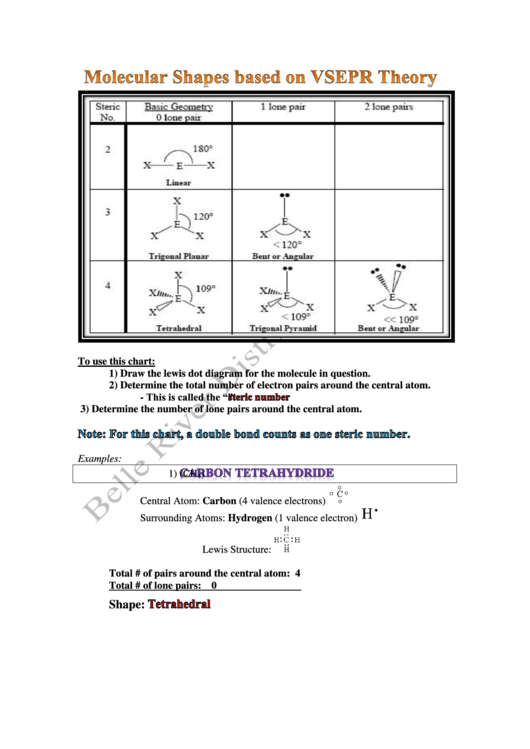
Molecular Shapes Based On Vsepr Theory
ADVERTISEMENT
To use this chart:
1) Draw the lewis dot diagram for the molecule in question.
2) Determine the total number of electron pairs around the central atom.
- This is called the “
”.
3) Determine the number of lone pairs around the central atom.
Examples:
1)
(CH
)
4
Central Atom: Carbon (4 valence electrons)
Surrounding Atoms: Hydrogen (1 valence electron)
Lewis Structure:
Total # of pairs around the central atom: 4
Total # of lone pairs:
0
Shape:
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2








