Ap Chemistry Worksheet - Dr. Kathleen Gorski, Wilbraham And Monson Acaddemy Page 16
ADVERTISEMENT
8.7 The partial Lewis structure that follows is for a hydrocarbon molecule. In the full Lewis
structure, each carbon atom satisfies the octet rule, and there are no unshared electron pairs in
the molecule. The carbon–carbon bonds are labeled 1, 2, and 3. (a) Determine where the
hydrogen atoms are in the molecule.(b) Rank the carbon–carbon bonds in order of increasing
bond length. (c) Rank the carbon–carbon bonds in order of increasing bond enthalpy. [Sections
8.3 and 8.8]
8.13 Write the Lewis symbol for atoms of each of the following elements: (a) Al, (b) Br, (c) Ar,
(d) Sr.
8.26 Explain the following trends in lattice energy: (a) NaCl > RbBr > CsBr; (b) BaO > KF; (c)
SrO > SrCl
.
2
8.41 Which of the following bonds are polar: (a) B – F, (b) Cl – Cl, (c) Se – O, (d) H – I? Which
is the more electronegative atom in each polar bond?
8.43 (a) From the data in Table 8.3, calculate the effective charges on the H and Br atoms of the
HBr molecule in units of the electronic charge, e. (b) Compare your answers to part (a) with
those in Sample Exercise 8.5 for the HCl molecule. Can you explain why the values are
different?
8.51 Write Lewis structures that obey the octet rule for each of the following, and assign oxidation
numbers and formal charges to each atom: (a) OCS, (b) SOCl
(S is bonded to the two Cl atoms
2
-
and to the O), (c) BrO
(d) HClO
(H is bonded to O).
3
2
+
-
-
8.56 Based on Lewis structures, predict the ordering of N – O bond lengths in NO
, NO
, NO
.
2
3
8.63 Draw the Lewis structures for each of the following ions or molecules. Identify those that do
2-,
3-,
not obey the octet rule, and explain why they do not: (a) SO
(b) AlH
, (c) N
(d) CH
Cl
, (e)
3
3
2
2
SbF
.
5
8.70 Using Table 8.4, estimate for the following gas-phase reactions:
16
423 Main Street, Wilbraham, MA 01095-1715 · phone: 413.596.6811 · fax: 413.596.4108 ·
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4 5
5 6
6 7
7 8
8 9
9 10
10 11
11 12
12 13
13 14
14 15
15 16
16 17
17 18
18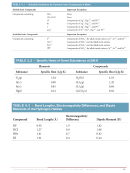 19
19 20
20 21
21 22
22 23
23 24
24








