Ap Chemistry Worksheet - Dr. Kathleen Gorski, Wilbraham And Monson Acaddemy Page 3
ADVERTISEMENT
2.3 Four of the boxes in the following periodic table are colored. Which of these are metals and
which are nonmetals? Which one is an alkaline earth metal? Which one is a noble gas?
[Section 2.5]
2.5 Which of the following diagrams most likely represents an ionic compound, and which
represents a molecular one? Explain your choice. [Sections 2.6 and 2.7]
2.7 Five of the boxes in the following periodic table are colored. Predict the charge on the ion
associated with each of these elements. [Section 2.7]
2.15 How did Rutherford interpret the following observations made during his α-particle
scattering experiments? (a) Most α particles were not appreciably deflected as they passed
through the gold foil. (b) A few particles were deflected at very large angles. (c) What
differences would you expect if beryllium foil were used instead of gold foil in the α-particle
scattering experiment?
-8
2.18 An atom of rhodium (Rh) has a diameter of about 2.7 x 10
cm. (a) What is the radius of a
rhodium atom in angstroms (Å) and in meters (m)? (b) How many Rh atoms would have to be
placed side by side to span a distance of 6.0 µm? (c) If you assume that the Rh atom is a
3
sphere, what is the volume in m
of a single atom?
2.26 Fill in the gaps in the following table, assuming each column represents a neutral atom.
65
Symbol
Zn
Protons
38
92
Neutrons
58 49
Electrons
38 36
Mass no.
81 235
3
423 Main Street, Wilbraham, MA 01095-1715 · phone: 413.596.6811 · fax: 413.596.4108 ·
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4 5
5 6
6 7
7 8
8 9
9 10
10 11
11 12
12 13
13 14
14 15
15 16
16 17
17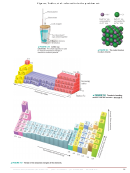 18
18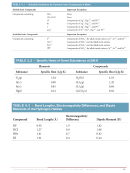 19
19 20
20 21
21 22
22 23
23 24
24








