Ap Chemistry Worksheet - Dr. Kathleen Gorski, Wilbraham And Monson Acaddemy Page 5
ADVERTISEMENT
3.4 The following diagram represents the collection of CO
and H
O molecules formed by
2
2
complete combustion of a hydrocarbon. What is the empirical formula of the hydrocarbon?
[Section 3.2].
3.8 Nitrogen monoxide and oxygen react to form nitrogen dioxide. Consider the mixture of NO
and O
shown in the accompanying diagram. The blue spheres represent N, and the red ones
2
represent O. (a) Draw a representation of the product mixture, assuming that the reaction goes
to completion. What is the limiting reactant in this case? (b) How many NO
molecules would
2
you draw as products if the reaction had a percent yield of 75%? [Section 3.7]
3.12 Balance the following equations:
(a) Li (s) + N
(g) ! Li
N (s)
2
3
(b) TiCl
(l) + H
O (l) ! TiO
(s) + HCl (aq)
4
2
2
(c) NH
NO
(s) ! N
(g) + O
(g) + H
O (g)
4
3
2
2
2
(d) Ca
P
(s) + H
O (l) ! Ca(OH)
(aq) + PH
(g)
3
2
2
2
3
(aq) ! Al
(e) Al(OH)
(s) + H
SO
(SO
)
(aq) + H
O (l)
3
2
4
2
4
3
2
(aq) ! Ag
(f) AgNO
(aq) + Na
CO
NO
(s) + Na
CO
(aq)
3
2
3
2
3
2
3
(g) C
H
NH
(g) + O
(g) ! CO
(g) + H
O (g) + N
(g)
2
5
2
2
2
2
2
3.20 Balance the following equations and indicate whether they are combination, decomposition,
or combustion reactions:
(a) PbCO
(s) ! PbO (s) + CO
(g)
3
2
(b) C
H
(s) + O
! CO
(g) + H
O (g)
2
4
2
2
2
(c) Mg (s) + N
(g) ! Mg
N
(s)
2
3
2
(d) C
H
O
(l) + O
! CO
(g) + H
O (g)
7
8
2
2
2
2
(g) ! AlCl
(e) Al(s) + Cl
(s)
2
3
3.21 Determine the formula weights of each of the following compounds: (a) nitric acid, HN
; (b)
3
KMnO
; (c) Ca
(PO
)
; (d) quartz, SiO
; (e) gallium sulfide, (f) chromium(III) sulfate, (g)
4
3
4
2
2
phosphorus trichloride.
5
423 Main Street, Wilbraham, MA 01095-1715 · phone: 413.596.6811 · fax: 413.596.4108 ·
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4 5
5 6
6 7
7 8
8 9
9 10
10 11
11 12
12 13
13 14
14 15
15 16
16 17
17 18
18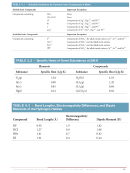 19
19 20
20 21
21 22
22 23
23 24
24








