Molecular Orbital Theory, Valence Bond Theory And Hybridization Worksheets Page 2
ADVERTISEMENT
Chemistry 121 Problem set VI solutions - 2
e) Nonpolar. ∆EN (O-C) = 1. The 2 opposing ∆EN (O-C) vectors cancel.
O
C
O
IF
5
a) Lewis structure is first structure and has an extra lone pair on the central atom
b) VSEPR
5 bp + 1 lp = 6
shape is octahedral
c) Molecular shape is square pyramid (second structure).
3
2
d) Hybridization is sp
d
(VSEPR 6 pairs on central atom so need 6 orbitals)
e) Weakly polar. ∆EN (F-I) = 1.5. The 4 ∆EN (F-I) vectors in the square plane will cancel. The ∆EN (F-I)
vector will come close to cancelling with the lone pair vector.
F
F
F
F
F
F
I
I
F
F
F
F
SF
4
a) Lewis structure is first structure and has an extra lone pair on the central atom
b) VSEPR
4 bp + 1 lp = 5
shape is trigonal bipyramidal
c) Molecular shape is seesaw (second structure).
3
d) Hybridization is sp
d (VSEPR 5 pairs on central atom so need 5 orbitals)
e) Weakly polar. ∆EN (F-S) = 1.5. The 2 ∆EN (F-S) vectors in the vertical plane will cancel. The resultant
of the two ∆EN(F-S) vectors will come close to cancelling with the lone pair vector in the horizontal
triangular plane.
F
F
F
F
S
F
F
F
F
ICl
3
a) Lewis structure is first structure and has two extra lone pairs on the central atom
b) VSEPR
3 bp + 2 lp = 5
shape is trigonal bipyramidal
c) Molecular shape is T shaped (second structure).
3
d) Hybridization is sp
d (VSEPR 5 pairs on central atom so need 5 orbitals)
e) Polar. ∆EN (Cl-I) = 0.5. The 2 ∆EN (F-S) vectors in the vertical plane will cancel. The resultant of the
two lone pair vectors will be much greater than the ∆EN (F-S) vector in the horizontal triangular plane.
Cl
Cl
Cl
I
Cl
Cl
Cl
XeF
2
a) Lewis structure is first structure and has three extra lone pairs on the central atom
b) VSEPR
2 bp + 3 lp = 5
shape is trigonal bipyramidal
c) Molecular shape is linear, lone pairs go into the horizintal trigonal plane. (second structure).
3
d) Hybridization is sp
d (VSEPR 5 pairs on central atom so need 5 orbitals)
e) Nonpolar. ∆EN (F-Xe) = 1.4. The 2 ∆EN (F-Xe) vectors in the vertical plane will cancel. The 3 lone pair
vectors in the horizontal triangular plane will cancel.
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4 5
5 6
6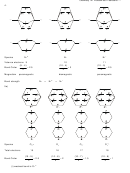 7
7 8
8








