Chemistry I Atomic Bonding Practice Quiz With Answer Key - Mr. Scott Page 12
ADVERTISEMENT
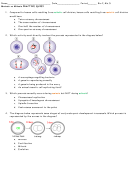
62)
What is the Lewis structure for carbon tetraiodide, which contains one carbon atom and
four iodine atoms?
a) A
b) B
c) C
d) D
63)
Bonding in molecules or ions that cannot be correctly represented by a single Lewis
structure is
a) covalent bonding.
b) resonance.
d) double bonding.
c) single bonding.
64)
Resonance structures are also called
a) dichotomous structures.
b) Lewis structures.
c) molecular structures.
d) resonance hybrids.
65)
To indicate resonance, a __________ is placed between a molecule's resonance structures.
a) double-headed arrow
b) single-headed arrow
c) series of dots
d) Lewis structure
66)
Chemists once believed that a molecule that contains a single bond and a double bond
split its time existing as one of these two structures. This effect became known as
a) alternation.
b) resonance.
c) Lewis structure.
d) single-double bonding.
- 12 -
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4 5
5 6
6 7
7 8
8 9
9 10
10 11
11 12
12 13
13 14
14 15
15 16
16 17
17 18
18 19
19 20
20 21
21 22
22 23
23 24
24 25
25 26
26 27
27 28
28 29
29 30
30 31
31 32
32 33
33 34
34