Chemistry I Atomic Bonding Practice Quiz With Answer Key - Mr. Scott Page 21
ADVERTISEMENT
112)
VSEPR theory is a model for predicting
a) the strength of metallic
b) the shape of molecules.
bonds.
c) lattice energy values.
d) ionization energy.
113)
The concept that electrostatic repulsion between electron pairs surrounding an atom causes
these pairs to be separated as far as possible is the foundation of
a) VSEPR theory.
b) the hybridization model.
d) Lewis theory.
c) the electron-sea model.
114)
According to VSEPR theory, the electrostatic repulsion between electron pairs
surrounding an atom causes
a) an electron sea to form.
b) positive ions to form.
c) these pairs to be separated
d) light to reflect.
as far as possible.
115)
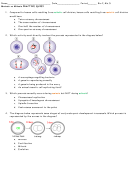
According to VSEPR theory, the shape of an AB
molecule is
3
a) trigonal planar.
b) tetrahedral.
c) linear.
d) bent.
116)
According to VSEPR theory, the structure of the ammonia molecule, NH
, is
3
a) linear.
b) bent.
c) pyramidal.
d) tetrahedral.
117)
Use VSEPR theory to predict the shape of the hydrogen chloride molecule, HCl.
a) tetrahedral
b) linear
c) bent
d) trigonal planar
- 21 -
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4 5
5 6
6 7
7 8
8 9
9 10
10 11
11 12
12 13
13 14
14 15
15 16
16 17
17 18
18 19
19 20
20 21
21 22
22 23
23 24
24 25
25 26
26 27
27 28
28 29
29 30
30 31
31 32
32 33
33 34
34