Chemistry I Atomic Bonding Practice Quiz With Answer Key - Mr. Scott Page 22
ADVERTISEMENT
118)
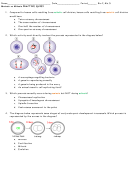
Use VSEPR theory to predict the shape of the magnesium hydride molecule, MgH
.
2
a) tetrahedral
b) linear
c) bent
d) octahedral
119)
Use VSEPR theory to predict the shape of the carbon tetraiodide molecule, CI
.
4
a) tetrahedral
b) linear
c) bent
d) trigonal planar
120)
Use VSEPR theory to predict the shape of the chlorate ion, ClO
3 –
.
a) trigonal planar
b) octahedral
d) bent
c) trigonal pyramidal
121)
Use VSEPR theory to predict the shape of the hydrogen sulfide molecule, H
S.
2
a) tetrahedral
b) linear
c) bent
d) octahedral
122)
Use VSEPR theory to predict the shape of carbon dioxide, CO
.
2
a) tetrahedral
b) linear
c) bent
d) octahedral
123)
The hybridized orbitals responsible for the bent shape of the water molecule are
b) ps
1
.
a) 1s
2
2s
2
.
c) sp
3
.
d) 2s
2
sp
2
.
- 22 -
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4 5
5 6
6 7
7 8
8 9
9 10
10 11
11 12
12 13
13 14
14 15
15 16
16 17
17 18
18 19
19 20
20 21
21 22
22 23
23 24
24 25
25 26
26 27
27 28
28 29
29 30
30 31
31 32
32 33
33 34
34