Chapter 12 - Structure Determination: Mass Spectrometry And Infrared Spectroscopy Page 15
ADVERTISEMENT
Bond Properties
The natural frequency of vibration of a bond is given by the
equation:
where µ = m
m
/(m
+m
) (termed the 'reduced mass'), and c
1
2
1
2
is the velocity of light.
K is a constant that varies from one bond to another. Force
constants for triple bonds are three times those of single
bonds.
Stronger bonds have a large force constant K and vibrate at
higher frequencies
Bonds between atoms of higher masses vibrate at lower
frequencies than bonds between lighter atoms.
15
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4 5
5 6
6 7
7 8
8 9
9 10
10 11
11 12
12 13
13 14
14 15
15 16
16 17
17 18
18 19
19 20
20 21
21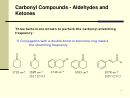 22
22 23
23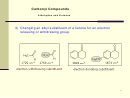 24
24 25
25 26
26 27
27 28
28 29
29 30
30 31
31 32
32 33
33 34
34 35
35 36
36 37
37 38
38 39
39 40
40 41
41 42
42 43
43 44
44








