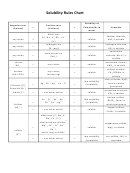
Solubility Rules Made Easy
ADVERTISEMENT
SolubilityRules Made Easy
Having trouble memorizing your solubility rules? This is the hand out for you. These
are two different ways that will help and make your life a little easier.
-
The first is an acronym: “CASH N’ Gia.” All these things are soluble unless
they are found with certain elements.
-
The second is a list of bullet points if you do not like the acronym. The best
way to remember these is pure memorization. There is no short cut!
All Soluble
Except With
C
hlorates
A
cetates
C
B
S
H
A
P
(CBS HAPpy)
S
ulfates
a
a
r
g
g
b
H
H
A
P
(HAPpy)
alogens
g
g
b
Simple Solubility Rules:
N’
itrates
-
-Nitrate (NO
) salts are soluble.
3
+
-Alkali (group 1A) salts and NH
are
G
4
roup (IA)
soluble.
-
-
-
-Cl
, Br
, and I
salts are soluble (NOT
i
+
2+
2+
Ag
, Pb
, Hg
)
2
-Sulfate salts are soluble (NOT BaSO
,
a
4
PbSO
, HgSO
, CaSO
)
4
4
4
−
-OH
salts are only slightly soluble
(NaOH, KOH are soluble,
Ba(OH)
,Ca(OH)
are marginally
2
2
soluble)
2−
2−
2−
3−
-S
, CO
, CrO
, PO
salts are
3
4
4
insoluble.
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1