Naming Compounds Flowchart Page 2
ADVERTISEMENT
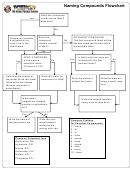
Naming Compounds Flowchart
Does any substance
end in -ate, -ite, or is
Yes
No
any substance
hydroxide or
ammonium?
Are there any
Substance contains
No
Yes
prefixes? (see table
a polyatomic ion.
below).
Look it up in the
table below.
IONIC COMPOUND
Write the first element
Look up the charge of
abbreviation; convert the
the cation (+ charge)
prefix to a subscript
number written AFTER the
chemical abbreviation. If
the first element does not
Look up the charge of
have a prefix, no number is
the anion (- charge)
required (there’s just one).
Write the second element
abbreviation; convert the
Adjust quantity of each
prefix to a subscript (if it’s
so that the total charge
mono, just leave it). Write
is zero. Make sure to
the number as a subscript
keep polyatomic ions as
after the chemical
a group. If more than
abbreviation.
one polyatomic ion is
needed, place it in
parenthesis.
The quantity of the
Common Prefixes
cation and anion are
for Covalent Compounds
Common Polyatmic Ions
written as a subscript
1 - mono
+
Ammonium NH
after the chemical
4
2 - di
-
Hydroxide OH
abbreviation.
3 - tri
-
Nitrate NO
3
4 - tetra
-2
Carbonate CO
5 - penta
3
-2
Sulfate SO
6 - hexa
4
-3
Phosphate PO
7 - hepta
4
8 - octa
9 - nona
10 - deca
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1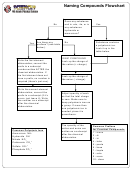 2
2