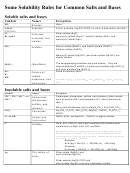
Common Cations, Anions, Acids, Salts And Hydrate Nomenclature Page 2
ADVERTISEMENT
Common Covalent Binary Inorganic Compounds
# of atoms Prefix
Common Examples
(element closest to fluorine goes on right)
1
Mono
H
Hydrogen
N
Nitrogen
2
2
2
Di
O
Oxygen
NH
Ammonia
2
3
3
Tri
O
Ozone
NO
Nitrogen monoxide (Nitric Oxide)
3
4
Tetra
H
O
Water (Dihydrogen Monoxide)
NO
Nitrogen dioxide
2
2
5
Penta
F
Fluorine
N
O
Dinitrogen monoxide (Nitrous oxide)
2
2
6
Hexa
HF
Hydrogen fluoride
N
O
Dinitrogen dioxide
2
2
7
Hepta
Cl
Chlorine
N
O
Dinitrogen tetroxide
2
2
4
8
Octa
HCl
Hydrogen chloride
CO
Carbon monoxide
9
Nona
Br
Bromine
CO
Carbon dioxide
2
2
10
Deca
I
Iodine
CCl
Carbon tetrachloride
2
4
Organic Nomenclature and Symbolism
(Other group prefixes)(longest chain prefix)(highest bond root)(most important group suffix)
Bond Order Name Drawn
Root
Formula
Carbon
Chain Prefix
Carbon has 4 bonds
1
Single
C-C
ane
C
H
In formula:
#
Systematic
Common
**
n
2n+2
Groups -1 H
2
Double C=C
ene
C
H
1
Methyl
Formyl
n
2n
Other C-C bonds -2 H
3
Triple
C≡C
yne
C
H
2
Ethyl
Acetyl
n
2n-2
3
Propyl
Propionyl
Group Name
Drawn
Prefix
Suffix
4
Butyl
Butyryl
Amine
-NH
Amino
amine
5
Pentyl
Valeryl
2
Ammonium ion
-NH
ammonium ion
6
Hexyl
Caproyl
+
3
7
Heptyl
Enanthyl
Carboxylic acid
Carboxyl
oic acid
*
or -COOH or -CO
H
8
Octyl
Caprylyl
2
9
Nonyl
Pelargonyl
Carboxylate ion
oate ion
*
or -COO
or -CO
-
-
10
Decyl
Capryl
2
Alcohol
-OH
Hydroxy
ol
Drop ‘yl’ from prefix for longest chain
-F
Fluoro
-Cl
Chloro
Include carbon in chain prefix
*
Halogen
-Br
Bromo
Don’t use bond root (names only ane)
**
-I
Iodo
Drop ‘o’ from carboxyl groups
Aromatic
Phenyl
(‘ic’ and ‘ate’)
or C
H
or -Ф or -Ph
6
5
Examples
Name
Formula
Systematic Name
Common Name
Formula
Name
Formula
Methane
CH
Methanoic acid
Formic acid
HCO
H
1,2-Dichloroethane
C
H
Cl
4
2
2
4
2
Ethane
C
H
Ethanoic acid
Acetic acid
CH
CO
H
Methylamine
CH
NH
2
6
3
2
3
2
Propane
C
H
Propanoic acid
Propionic acid
C
H
CO
H
Methylammonium ion
CH
NH
+
3
8
2
5
2
3
3
Butane
C
H
Butanoic acid
Butyric acid
C
H
CO
H
1,3-butadiene
C
H
4
10
3
7
2
4
6
Pentane
C
H
Pentanoic acid
Valeric acid
C
H
CO
H
Hydroxyethanoic acid
HOCH
CO
H
5
12
4
9
2
2
2
Methanol
CH
OH
Methanoate ion
Formate ion
HCO
Phenol
C
H
OH
-
3
2
6
5
Ethanol
C
H
OH
Ethanoate ion
Acetate ion
CH
CO
-
2
5
3
2
Propanol
C
H
OH
Propanoate ion
Propionate ion
C
H
CO
-
Special Names
Formula
3
7
2
5
2
Butanol
C
H
OH
Butanoate ion
Butyrate ion
C
H
CO
Benzene
C
H
-
4
9
3
7
2
6
6
Pentanol C
H
OH
Pentanoate ion
Valerate ion
C
H
CO
Toluene
C
H
CH
-
5
11
4
9
2
6
5
3
Most Common Formula Representations (All represent ethanol)
Example
C
H
O
CH
CH
OH
2
6
3
2
Condensed Molecular
Name
Molecular Formula
Structural Formula
Line Formula
Formula
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2