Solubilities Of Inorganic Compounds In Water Page 4
ADVERTISEMENT
TABLE 2-122 Solubilities of Inorganic Compounds in Water at Various Temperatures (Concluded)
Solid
Substance
Formula
phase
0 °C
10 °C
20 °C
30 °C
40 °C
50 °C
60 °C
70 °C
80 °C
90 °C
100 °C
25
75
1
Sodium vanadate (meta)
NaVO
21.10
°
26.23
32.97
36.9
38.8
°
1
3
2
Stannous chloride
SnCl
83.9
269.8
15
2
°
2
3
sulfate
SnSO
19
18
3
4
4
Strontium acetate
Sr(C
H
O
)
4H
O
36.9
43.61
4
2
3
2
2
2
5
acetate
Sr(C
H
O
)
aH
O
42.95
41.6
39.5
37.35
36.24
36.10
36.4
5
2
3
2
2
2
6
chloride
SrCl
6H
O
43.5
47.7
52.9
58.7
65.3
72.4
81.8
6
2
2
7
chloride
SrCl
2H
O
85.9
90.5
100.8
7
2
2
8
nitrate
Sr(NO
)
1H
O
52.7
64.0
83.8
97.2
130.4
139
8
3
2
2
9
nitrate
Sr(NO
)
4H
O
40.1
70.5
9
3
2
2
10
nitrate
Sr(NO
)
88.6
90.1
93.8
96
98
100
10
3
2
11
sulfate
SrSO
0.0113
0.0114
0.0114
11
4
12
Sulfur dioxide, 760 mm†
SO
22.83
16.21
11.29
7.81
5.41
4.5
12
2
13
Thallium sulfate
Tl
SO
2.70
3.70
4.87
6.16
9.21
10.92
12.74
14.61
16.53
18.45
13
2
4
14
Thorium sulfate
Th(SO
)
9H
O
0.74
0.98
1.38
1.995
2.998
5.22
14
4
2
2
15
sulfate
Th(SO
)
8H
O
1.0
1.25
1.62
15
4
2
2
16
sulfate
Th(SO
)
6H
O
1.50
1.90
2.45
6.64
16
4
2
2
17
sulfate
Th(SO
)
4H
O
4.04
2.54
1.63
1.09
17
4
2
2
18
Zinc chlorate
ZnClO
6H
O
145.0
152.5
18
3
2
19
chlorate
ZnClO
4H
O
200.3
209.2
223.2
273.1
19
3
2
20
nitrate
Zn(NO
)
6H
O
94.78
118.3
20
3
2
2
21
nitrate
Zn(NO
)
3H
O
206.9
21
3
2
2
22
sulfate
ZnSO
7H
O
41.9
47
54.4
22
4
2
23
sulfate
ZnSO
6H
O
70.1
76.8
23
4
2
24
sulfate
ZnSO
1H
O
86.6
83.7
80.8
24
4
2
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3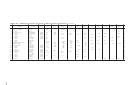 4
4








