Solubilities Of Inorganic Compounds In Water Page 2
ADVERTISEMENT
1
Calcium bicarbonate
Ca(HCO
)
16.15
16.60
17.05
17.50
17.95
18.40
1
3
2
2
chloride
CaCl
6H
O
59.5
65.0
74.5
102
2
2
2
3
chloride
CaCl
2H
O
136.8
141.7
147.0
152.7
159
3
2
2
18
26
4
fluoride
CaF
0.0016
°
0.0017
°
4
2
5
hydroxide
Ca(OH)
0.185
0.176
0.165
0.153
0.141
0.128
0.116
0.106
0.094
0.085
0.077
5
2
6
nitrate
Ca(NO
)
4H
O
102.0
115.3
129.3
152.6
195.9
6
3
2
2
7
nitrate
Ca(NO
)
3H
O
237.5
281.5
7
3
2
2
8
nitrate
Ca(NO
)
358.7
363.6
8
3
2
9
nitrite
Ca(NO
)
4H
O
62.07
76.68
9
2
2
2
10
nitrite
Ca(NO
)
2H
O
132.6
151.9
244.8
10
2
2
2
−4
−4
−4
−4
11
oxalate
CaC
O
6.7 × 10
6.8 × 10
9.5 × 10
14 × 10
11
2
4
at 13°
at 25°
at 50°
at 95°
12
sulfate
CaSO
2H
O
0.1759
0.1928
0.2090
0.2097
0.2047
0.1966
0.1619
12
4
2
13
Carbon dioxide, 760 mm ‡
CO
0.3346
0.2318
0.1688
0.1257
0.0973
0.0761
0.0576
0
13
2
14
monoxide, 760 mm ‡
CO
0.0044
0.0035
0.0028
0.0024
0.0021
0.0018
0.0015
0.0013
0.0010
0.0006
0
14
15
Cesium chloride
CsCl
161.4
174.7
186.5
197.3
208.0
218.5
229.7
239.5
250.0
260.1
270.5
15
16
nitrate
CsNO
9.33
14.9
23.0
33.9
47.2
64.4
83.8
107.0
134.0
163.0
197.0
16
3
17
sulfate
Cs
SO
167.1
173.1
178.7
184.1
189.9
194.9
199.9
205.0
210.3
214.9
220.3
17
2
4
18
Chlorine, 760 mm ‡
Cl
1.46
0.980
0.716
0.562
0.451
0.386
0.324
0.274
0.219
0.125
0
18
2
19
Chromic anhydride
CrO
164.9
174.0
182.1
217.5
206.8
19
3
20
Cuprio chloride
CuCl
2H
O
70.7
73.76
77.0
80.34
83.8
87.44
91.2
99.2
107.9
20
2
2
21
nitrate
Cu(NO
)
6H
O
81.8
95.28
125.1
21
3
2
2
22
nitrate
Cu(NO
)
3H
O
159.8
178.8
207.8
22
3
2
2
23
sulfate
CuSO
5H
O
14.3
17.4
20.7
25
28.5
33.3
40
55
75.4
23
4
2
24
sulfide
CuS
3.3 × 10
−5
24
at 18°
25
Cuprous chloride
CuCl
1.52
25
25
°
26
Ferric chloride
FeCl
74.4
81.9
91.8
315.1
525.8
535.7
26
3
27
Ferrous chloride
FeCl
4H
O
64.5
73.0
77.3
82.5
88.7
100
27
2
2
28
chloride
FeCl
105.3
105.8
28
2
29
nitrate
Fe(NO
)
6H
O
71.02
83.8
165.6
29
3
2
2
30
sulfate
FeSO
7H
O
15.65
20.51
26.5
32.9
40.2
48.6
30
4
2
31
sulfate
FeSO
1H
O
50.9
43.6
37.3
31
4
2
32
Hydrobromic acid, 760 mm
HBr
221.2
210.3
198
171.5
130
32
33
Hydrochloric acid, 760 mm
HCl
82.3
67.3
63.3
59.6
56.1
33
34
Iodine
I
0.029
0.04
0.056
0.078
34
2
25
35
Lead acetate
Pb(C
H
O
)
3H
O
55.04
°
35
2
3
2
2
2
36
bromide
PbBr
0.4554
0.85
1.15
1.53
1.94
2.36
3.34
4.75
36
2
37
carbonate
PbCO
0.00011
37
3
38
chloride
PbCl
0.6728
0.99
1.20
1.45
1.70
1.98
2.62
3.34
38
2
−6
39
chromate
PbCrO
7 × 10
39
4
40
fluoride
PbF
0.060
0.064
0.068
40
2
41
nitrate
Pb(NO
)
38.8
48.3
56.5
66
75
85
95
115
38.8
41
3
2
42
sulfate
PbSO
0.0028
0.0035
0.0041
0.0049
0.0056
42
4
43
Magnesium bromide
MgBr
6H
O
91.0
94.5
96.5
99.2
101.6
104.1
107.5
113.7
120.2
43
2
2
44
chloride
MgCl
6H
O
52.8
53.5
54.5
57.5
61.0
66.0
73.0
44
2
2
18
45
hydroxide
Mg(OH)
0.0009
°
45
2
46
nitrate
Mg(NO
)
6H
O
66.55
84.74
137.0
46
3
2
2
47
sulfate
MgSO
7H
O
30.9
35.5
40.8
45.6
47
4
2
48
sulfate
MgSO
6H
O
40.8
42.2
44.5
45.3
50.4
53.5
59.5
64.2
69.0
74.0
48
4
2
49
sulfate
MgSO
1H
O
62.9
68.3
49
4
2
50
Manganous sulfate
MnSO
7H
O
53.23
60.01
50
4
2
51
sulfate
MnSO
5H
O
59.5
62.9
67.76
51
4
2
52
sulfate
MnSO
4H
O
64.5
66.44
68.8
72.6
52
4
2
53
sulfate
MnSO
1H
O
58.17
55.0
52.0
48.0
42.5
34.0
53
4
2
54
Mercurous chloride
HgCl
0.00014
0.0002
0.0007
54
55
Molybdic oxide
MoO
2H
O
0.138
0.264
0.476
0.687
1.206
2.055
2.106
55
3
2
56
Nickel chloride
NiCl
6H
O
53.9
59.5
64.2
68.9
73.3
78.3
82.2
85.2
87.6
56
2
2
57
nitrate
Ni(NO
)
6H
O
79.58
96.31
122.2
57
3
2
2
58
nitrate
Ni(NO
)
3H
O
163.1
169.1
235.1
58
3
2
2
59
sulfate
NiSO
7H
O
27.22
32
42.46
59
4
2
60
sulfate
NiSO
6H
O
50.15
54.80
59.44
63.17
76.7
60
4
2
61
Nitric oxide, 760 mm
NO
0.00984
0.00757
0.00618
0.00517
0.00440
0.00376
0.00324
0.00267
0.00199
0.00114
0
61
62
Nitrous oxide
N
O
0.1705
0.1211
62
2
*By N. A. Lange; abridged from “Table of Solubilities of Inorganic Compounds in Water at Various Temperatures” in Lange’s Handbook of Chemistry, 10th ed., McGraw-Hill, New York, 1961 (except for NaCl, which is from CRC Handbook of Chemistry
and Physics, 86th ed., CRC Press, 2005). For tables of the solubility of gases in water at various temperatures, Atack (Handbook of Chemical Data, Reinhold, New York, 1957) gives values at closer temperature intervals, usually 1 or 5 °C, than are tabulated
here. For materials marked by ‡, additional data are given in tables subsequent to this one. For the solubility of various hydrocarbons in water at high pressures see J. Chem. Eng. Data, 4, 212 (1959).
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3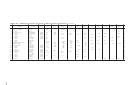 4
4








