Solubilities Of Inorganic Compounds In Water Page 3
ADVERTISEMENT
TABLE 2-122 Solubilities of Inorganic Compounds in Water at Various Temperatures (Continued)
Solid
Substance
Formula
phase
0 °C
10 °C
20 °C
30 °C
40 °C
50 °C
60 °C
70 °C
80 °C
90 °C
100 °C
1
Potassium acetate
KC
H
O
1aH
O
216.7
233.9
255.6
283.8
323.3
1
2
3
2
2
2
acetate
KC
H
O
O
337.3
350
364.8
380.1
396.3
2
aH
2
3
2
2
3
alum
K
SO
(SO
)
24H
O
3.0
4.0
5.9
8.39
11.70
17.00
24.75
40.0
71.0
109.0
3
2
4
⋅Al
2
4
3
2
4
bicarbonate
KHCO
22.4
27.7
33.2
39.1
45.4
60.0
4
3
5
bisulfate
KHSO
36.3
51.4
67.3
121.6
5
4
6
bitartrate
KHC
H
O
0.32
0.40
0.53
0.90
1.32
1.83
2.46
4.6
6.95
6
4
4
6
7
carbonate
K
CO
2H
O
105.5
108
110.5
113.7
116.9
121.2
126.8
133.1
139.8
147.5
155.7
7
2
3
2
8
chlorate
KClO
3.3
5
7.4
10.5
14
19.3
24.5
38.5
57
8
3
9
chloride
KCl
27.6
31.0
34.0
37.0
40.0
42.6
45.5
48.3
51.1
54.0
56.7
9
10
chromate
K
CrO
58.2
60.0
61.7
63.4
65.2
66.8
68.6
70.4
72.1
73.9
75.6
10
2
4
11
dichromate
K
Cr
O
5
7
12
20
26
34
43
52
61
70
80
11
2
2
7
104
12
ferricyanide
K
Fe(CN)
31
36
43
50
60
66
82.6
12
3
6
13
hydroxide
KOH
2H
O
97
103
112
126
13
2
14
hydroxide
KOH
1H
O
140
178
14
2
15
nitrate
KNO
13.3
20.9
31.6
45.8
63.9
85.5
110.0
138
169
202
246
15
3
16
nitrite
KNO
278.8
298.4
334.9
412.8
16
2
17
perchlorate
KClO
0.75
1.05
1.80
2.6
4.4
6.5
9
11.8
14.8
18
21.8
17
4
18
permanganate
KMnO
2.83
4.4
6.4
9.0
12.56
16.89
22.2
18
4
19
persulfate†
K
S
O
†
†
1.62
2.60
4.49
7.19
9.89
19
2
2
8
20
sulfate
K
SO
7.35
9.22
11.11
12.97
14.76
16.50
18.17
19.75
21.4
22.8
24.1
20
2
4
21
thiocyanate
KCNS
177.0
217.5
21
−5
22
Silver cyanide
AgCN
2.2 × 10
22
23
nitrate
AgNO
122
170
222
300
376
455
525
669
952
23
3
24
sulfate
Ag
SO
0.573
0.695
0.796
0.888
0.979
1.08
1.15
1.22
1.30
1.36
1.41
24
2
4
25
Sodium acetate
NaC
H
O
3H
O
36.3
40.8
46.5
54.5
65.5
83
139
25
2
3
2
2
26
acetate
NaC
H
O
119
121
123.5
126
129.5
134
139.5
146
153
161
170
26
2
3
2
27
bicarbonate
NaHCO
6.9
8.15
9.6
11.1
12.7
14.45
16.4
27
3
28
carbonate
Na
CO
10H
O
7
12.5
21.5
38.8
28
2
3
2
29
carbonate
Na
CO
1H
O
50.5
48.5
46.4
45.8
45.5
29
2
3
2
30
chlorate
NaClO
79
89
101
113
126
140
155
172
189
230
30
8
31
chloride
NaCl
35.65
35.72
35.89
36.09
36.37
36.69
37.04
37.46
37.93
38.47
38.99
31
32
chromate
Na
CrO
10H
O
31.70
50.17
88.7
32
2
4
2
33
chromate
Na
CrO
4H
O
88.7
95.96
104
114.6
33
2
4
2
34
chromate
Na
CrO
123.0
124.8
125.9
34
2
4
35
dichromate
Na
Cr
O
2H
O
163.0
177.8
244.8
316.7
376.2
35
2
2
7
2
36
dichromate
Na
Cr
O
426.3
36
2
2
7
37
dihydrogen phosphate
NaH
PO
2H
O
57.9
69.9
85.2
106.5
138.2
37
2
4
2
38
dihydrogen phosphate
NaH
PO
1H
O
158.6
38
2
4
2
39
dihydrogen phosphate
NaH
PO
179.3
190.3
207.3
225.3
246.6
39
2
4
40
hydrogen arsenate
Na
HAsO
12H
O
7.3
15.5
26.5
37
47
65
85
40
2
4
2
41
hydrogen phosphate
Na
HPO
12H
O
1.67
3.6
7.7
20.8
41
2
4
2
42
hydrogen phosphate
Na
HPO
7H
O
51.8
42
2
4
2
43
hydrogen phosphate
Na
HPO
2H
O
80.2
82.9
88.1
92.4
102.9
43
2
4
2
44
hydrogen phosphate
Na
HPO
102.2
44
2
4
45
hydroxide
NaOH
4H
O
42
45
2
46
hydroxide
NaOH
3aH
O
51.5
46
2
47
hydroxide
NaOH
1H
O
109
119
129
145
174
47
2
48
hydroxide
NaOH
313
347
48
49
nitrate
NaNO
73
80
88
96
104
114
124
148
180
49
3
50
nitrite
NaNO
72.1
78.0
84.5
91.6
98.4
104.1
132.6
163.2
50
2
51
oxalate
Na
C
O
3.7
6.33
51
2
2
4
52
phosphate, tri-
Na
PO
12H
O
1.5
4.1
11
20
31
43
55
81
108
52
3
4
2
53
pyrophosphate
Na
P
O
10H
O
3.16
3.95
6.23
9.95
13.50
17.45
21.83
30.04
40.26
53
4
2
7
2
54
sulfate
Na
SO
10H
O
5.0
9.0
19.4
40.8
54
2
4
2
55
sulfate
Na
SO
7H
O
19.5
30
44
55
2
4
2
56
sulfate
Na
SO
48.8
46.7
45.3
43.7
42.5
56
2
4
57
sulfide
Na
S
9H
O
15.42
18.8
22.5
28.5
57
2
2
58
sulfide
Na
S
5aH
O
39.82
42.69
45.73
51.40
59.23
58
2
2
59
sulfide
Na
S
6H
O
36.4
39.1
43.31
49.14
57.28
59
2
2
60
sulfite
Na
SO
7H
O
13.9
20
26.9
36
60
2
3
2
61
sulfite
Na
SO
28
28.2
28.8
28.3
61
2
3
62
tetraborate
Na
B
O
10H
O
1.3
1.6
2.7
3.9
10.5
20.3
62
2
4
7
2
63
tetraborate
Na
B
O
5H
O
24.4
31.5
41
52.5
63
2
4
7
2
25
64
vanadate (meta)
NaVO
2H
O
15.3
°
30.2
68.4
64
8
2
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3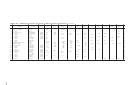 4
4








