Chemistry Worksheets Page 2
ADVERTISEMENT
+
-
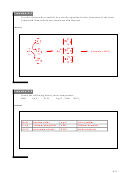
•
Na
and Cl
form NaCl = table salt
•
Strong bonds.
Polar and Non-polar in Solutions
•
Polar substances dissolve in polar solvents
•
Non-polar substances dissolve in non-polar solvents
•
Like dissolves like.
•
Polar = Hydrophilic (water loving)
•
Non-polar = Hydrophobic (water fearing)
•
Amphipathic molecule has both polar and non-polar parts. Bile salts and phospholipids.
Hydrogen Bonds
•
Bonds formed between H of one molecule to O or N of another molecule due to unequal sharing of electron pairs.
•
Weak bonds
•
Formed between water molecules
Chapter 2 Recap 1
•
All matter is formed of smallest units, with unique chemical properties, called -----------
•
# of ---------- determine the chemical substance (element).
•
# of ---------- determine the isotope.
•
Atomic Number is equal to # of ----------- in an atom.
•
Mass Number is sum of # of ---- and # of -------- in an atom
•
------------ bonds are formed due to complete transfer of electrons
•
----------- bonds are formed due to sharing of electrons
•
# of electrons in outer shell determine --------
----------
•
---------- bonds are very weak bonds formed by water molecules
Acids, Bases and pH scale
+
•
Acids produce H
ions
+
•
Bases accept H
ions
+
+
•
Salts produce ions other than H
or OH
ions
+
+
•
pH scale measures the [H
] = chemical concentration of H
ions
+
+
•
High [H
] means low pH and low [H
] means high pH
•
pH scale varies from 0 – 14
•
pH = 0 strongest acid; pH = 14 is strongest base; pH = 7 is neutral belongs to chemically pure water
•
Buffers are chemicals that resist fast changes in pH
Chapter 2 Recap 2
+
•
---------- release H
•
pH scale has a range of --- to ----
•
pH of water is ----
•
------------ resist fast changes to pH of a system.
+
+
•
A substance producing ions other than H
or OH
is a ------------
•
------- molecules form spherical clusters in water called Micelle.
Carbohydrates
•
Monosaccharides formed of 1 unit – Glucose
•
Disaccharides formed of 2 units; glucose and fructose form Sucrose – table sugar; other important disaccharides are Maltose
– formed of 2 glucose molecules and Lactose formed of glucose and galactose molecules
•
Polysaccharides are formed of many units. Starch and glycogen are formed of α-glucose molecules; Cellulose is formed of
β-glucose molecules
Lipids
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3