Elimination Reactions And Alkene Synthesis - Organic Chemistry Worksheet With Answers Page 10
ADVERTISEMENT
29) C
30) This is a typical example of a simple, multistep synthesis (in this case only two steps). This
tests your ability to use previously learned reactions (e.g. from ch. 4) to design a synthesis
towards a particular product, in this case cyclopentene. The last step is an elimination reaction.
Any strong base combination will serve the same purpose as NaOH and acetone.
Br
Br
2
NaOH
hv
acetone
A
B
C
31) Similar to the previous problem, but this time Hoffman’s product is desired. A bulky base
must be used in the last step, such as t-butoxide ion.
-
Br
t-BuO
2
t-BuOH
h
n
Br
Hoffman's
C
A
B
product
32) Same as above, but this time Sayteff’s product is desired. A small base must be used in the
last step.
Br
NaOH
2
h
n
acetone
Br
Saytzeff's
C
A
B
product
33) D
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2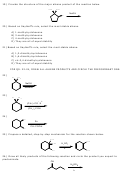 3
3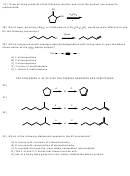 4
4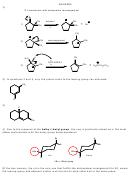 5
5 6
6 7
7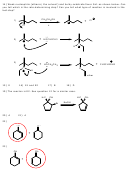 8
8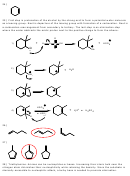 9
9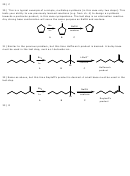 10
10








