Elimination Reactions And Alkene Synthesis - Organic Chemistry Worksheet With Answers Page 4
ADVERTISEMENT
27) Draw all likely products of the following reaction and circle the product you expect to
predominate.
CH
3
H
SO
2
4
OH
D
28) Which base, ammonia (NH 3 ) or triethylamine [(CH 3 CH 2 ) 3 N], would be more effective to use
for the following conversion?
Base
Cl
29) Which compound would undergoe dehydrohalogenation with strong base to give the alkene
shown below as the only alkene product?
CH
CH
C
CH
CH
3
2
3
H
A) 1-chloropentane
B) 2-chloropentane
C) 3-chloropentane
D) 1-chloro-2-methylbutane
E) 1-chloro-3-methylbutane
FOR SYNTHESES # 30-32 GIVE THE MISSING REAGENTS AND STRUCTURES.
30)
A
C
B
31)
A
C
B
32)
A
C
B
33) Which of the following statements applies to the E2 mechanism?
A) It occurs with inversion of stereochemistry.
B) It occurs with racemization of stereochemistry.
C) It proceeds through the more stable carbocation intermediate.
D) The C-H and C-X bonds that break must be anti.
E) Use of a bulky base gives the more highly substituted alkene product.
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2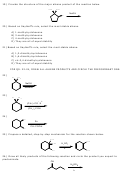 3
3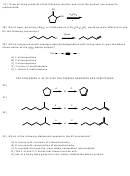 4
4 5
5 6
6 7
7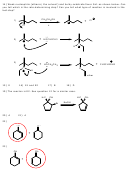 8
8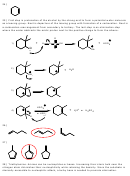 9
9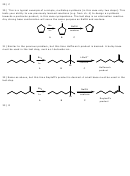 10
10








