Elimination Reactions And Alkene Synthesis - Organic Chemistry Worksheet With Answers Page 7
ADVERTISEMENT
10)
2
3
2
4
6
2
4
6
8
1
8
3
1
5
3
7
1
5
7
8
6
4
5
7
(E)-3-methyloct-3-ene
(E)-3-methyloct-2-ene
(Z)-3-methyloct-2-ene
4
6
8
7
3
3
5
5
7
2
4
6
2
1
1
(Z)-3-methyloct-3-ene
2-Ethylheptene
11) E2. The molecule must rotate around the central cabon-carbon bond to aquire the
anticoplanar arrangement required for E2. This is a stereospecific reaction that results in
formation of the product where the phenyl groups are cis to each other.
H
C
H
3
Ph
Ph
12) Strong base and bulky substrate favor E2. Only carbon 6 has protons trans to the leaving
group. The pi bond can only form between carbons 1 and 6. Can you name the product by IUPAC
rules?
4
5
3
NaOCH
3
2
6
H
1
H
C
H
C
3
CH
OH
3
3
H
C
H
H
C
3
3
Cl
13) Strong base and bulky substrate favor E2 with preferential formation of Saytzeff’s product.
NaOCH
CH
2
3
CH
CH
OH
Cl
3
2
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2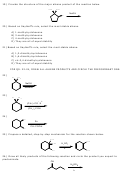 3
3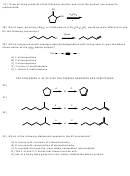 4
4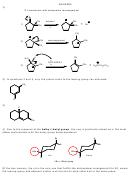 5
5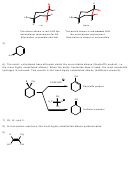 6
6 7
7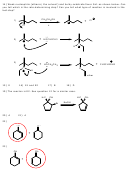 8
8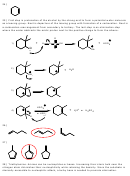 9
9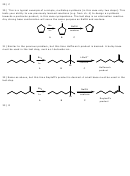 10
10








