Elimination Reactions And Alkene Synthesis - Organic Chemistry Worksheet With Answers Page 5
ADVERTISEMENT
ANSWERS
1)
E1 mechanism with carbocation rearrangement
Br
CH
ethanol
CH
3
3
+
Br
1)
CH
CH
3
3
D
CH
3
rearrangement
CH
CH
2)
3
3
CH
3
H
CH
H
C
3
3
HOCH
CH
2
3
CH
CH
+
H
OCH
CH
3)
3
3
2
2
3
2) In questions 2 and 3, only the proton trans to the leaving group can eliminate.
CH
3
D
3)
H
CH
3
4) Due to the presence of the bulky t-butyl group, the ring is practically locked up in the most
stable conformation with the bulky group being equatorial.
H
Br
Br
H
t-Bu
t-Bu
trans
cis
H
H
t-Bu = t-Butyl group
Of the two isomers, the cis is the only one that fulfills the anticoplanar arrangement for E2, where
the leaving group and adjacent proton must be anti to each other and in the same plane.
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2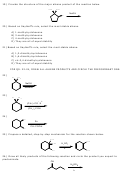 3
3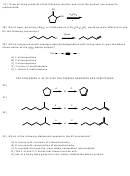 4
4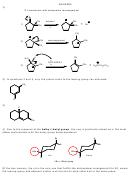 5
5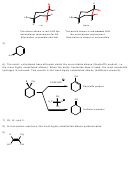 6
6 7
7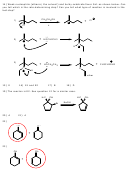 8
8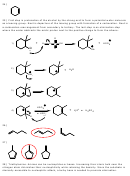 9
9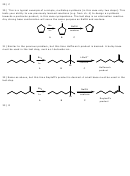 10
10








