Elimination Reactions And Alkene Synthesis - Organic Chemistry Worksheet With Answers Page 9
ADVERTISEMENT
24)
25) First step is protonation of the alcohol by the strong acid to form a potential water molecule
as a leaving group. Next is departure of the leaving group with formation of a carbocation. Next is
a carbocation rearrangement from secondary to tertiary. The last step is an elimination step
where the water abstracts the acidic proton next to the positive charge to form the alkene.
O
+
+
1)
HSO
H
O
S
OH
4
OH
O
OH
2
2)
+
H
O
2
OH
2
3)
HSO
4
+
+
or H
SO
4)
H
O
2
4
3
or H
O
2
H
26)
27)
28) Triethylamine. Amines can be nucleophiles or bases. Increasing their steric bulk near the
nitrogen atom diminishes their nucleophilicity while retaining the basicity. Since the substrate is
sterically accessible to nucleophilic attack, a bulky base is needed to promote elimination.
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2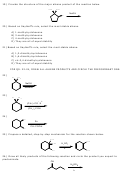 3
3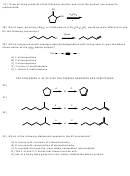 4
4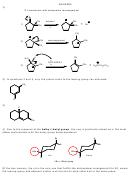 5
5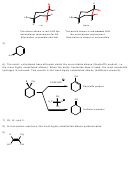 6
6 7
7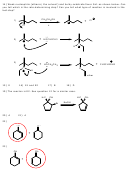 8
8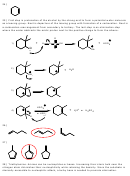 9
9 10
10








