Amino Acids Peptides And Proteins Page 43
ADVERTISEMENT
1195
24
Glossary
primary structure The covalently bonded structure of a protein; the sequence of amino acids,
together with any disulfide bridges. (p. 1188)
prion protein A protein infectious agent that is thought to promote misfolding and polymer-
ization of normal protein molecules, leading to amyloid plaques and destruction of nerve
tissue. (p. 1192)
prosthetic group The nonprotein part of a conjugated protein. Examples of prosthetic groups
are sugars, lipids, nucleic acids, and metal complexes. (p. 1188)
protein A biopolymer of amino acids. Proteins are polypeptides with molecular weights
higher than about 6000 amu. (p. 1171)
quaternary structure The association of two or more peptide chains into a composite protein.
(p. 1190)
random coil A type of protein secondary structure where the chain is neither curled into an
a-helix
nor lined up in a pleated sheet. In a globular protein, the kinks that fold the molecule into
its globular shape are usually segments of random coil. (p. 1190)
residue An amino acid unit of a peptide. (p. 1171)
Sanger method A method for determining the N-terminal amino acid of a peptide. The pep-
tide is treated with 2,4-dinitrofluorobenzene (Sanger’s reagent), then completely hydrolyzed.
The derivatized amino acid is easily identified, but the rest of the peptide is destroyed in the
hydrolysis. (p. 1178)
secondary structure The local hydrogen-bonded arrangement of a protein. The secondary
structure is generally the
a
helix, pleated sheet, or random coil. (p. 1189)
sequence As a noun, the order in which amino acids are linked together in a peptide. As a verb,
to determine the sequence of a peptide. (p. 1176)
simple proteins Proteins composed of only amino acids (having no prosthetic groups).
(p. 1188)
solid-phase peptide synthesis A method in which the C-terminal amino acid is attached to a
solid support (polystyrene beads) and the peptide is synthesized in the
C : N
direction by suc-
cessive coupling of protected amino acids. When the peptide is complete, it is cleaved from the
solid support. (p. 1183)
solution-phase peptide synthesis (classical peptide synthesis) Any of several methods in
which protected amino acids are coupled in solution in the correct sequence to give a desired
peptide. Most of these methods proceed in the
N : C
direction (p. 1181)
standard amino acids The 20
a-amino
acids found in nearly all naturally occurring proteins.
(p. 1155)
Strecker synthesis Synthesis of
a-amino
acids by reaction of an aldehyde with ammonia and
cyanide ion, followed by hydrolysis of the intermediate
a-amino
nitrile. (p. 1165)
O
NH
NH
2
3
+
H
O
H
O
2
3
R 9 C 9 H
R 9 C 9 H
R 9 C 9 H
NH
HCN
3
C # N
COOH
a-amino nitrile
a-amino acid
aldehyde
terminal residue analysis Sequencing a peptide by removing and identifying the residue at
the N terminus or at the C terminus. (p. 1176)
tertiary structure The complete three-dimensional conformation of a protein. (p. 1190)
transamination Transfer of an amino group from one molecule to another. Transamination is
a common method for the biosynthesis of amino acids, often involving glutamic acid as the
source of the amino group. (p. 1162)
zwitterion (dipolar ion) A structure with an overall charge of zero but having a positively
charged substituent and a negatively charged substituent. Most amino acids exist in zwitterionic
forms. (p. 1158)
O
O
N 9 CH 9 C 9 OH
N 9 CH 9 C 9 O
H
H
2
3
R
R
uncharged structure
dipolar ion, or zwitterion
(minor component)
(major component)
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4 5
5 6
6 7
7 8
8 9
9 10
10 11
11 12
12 13
13 14
14 15
15 16
16 17
17 18
18 19
19 20
20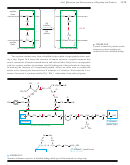 21
21 22
22 23
23 24
24 25
25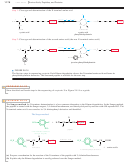 26
26 27
27 28
28 29
29 30
30 31
31 32
32 33
33 34
34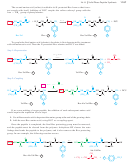 35
35 36
36 37
37 38
38 39
39 40
40 41
41 42
42 43
43 44
44 45
45 46
46 47
47