Amino Acids Peptides And Proteins Page 8
ADVERTISEMENT
1160
Amino Acids, Peptides, and Proteins
CHAPTER 24
24-4
An amino acid bears a positive charge in acidic solution (low pH) and a negative
charge in basic solution (high pH). There must be an intermediate pH where the amino
Isoelectric Points
acid is evenly balanced between the two forms, as the dipolar zwitterion with a net
charge of zero. This pH is called the isoelectric pH or the isoelectric point.
and Electrophoresis
−
−
OH
OH
N 9 CH 9 COOH
N 9 CH 9 COO
N 9 CH 9 COO
H
H
H
3
+
3
+
2
H
H
R
R
R
low pH
isoelectric pH
high pH
(cationic in acid)
(neutral)
(anionic in base)
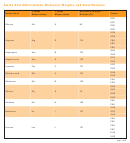
The isoelectric points of the standard amino acids are given in Table 24-2. Notice
that the isoelectric pH depends on the amino acid structure in a predictable way.
acidic amino acids:
aspartic acid (2.8), glutamic acid (3.2)
neutral amino acids:
(5.0 to 6.3)
basic amino acids:
lysine (9.7), arginine (10.8), histidine (7.6)
The side chains of aspartic acid and glutamic acid contain acidic carboxyl groups.
These amino acids have acidic isoelectric points around pH 3. An acidic solution is
needed to prevent deprotonation of the second carboxylic acid group and to keep the
amino acid in its neutral isoelectric state.
Basic amino acids (histidine, lysine, and arginine) have isoelectric points at pH
values of 7.6, 9.7, and 10.8, respectively. These values reflect the weak basicity of the
imidazole ring, the intermediate basicity of an amino group, and the strong basicity of
the guanidino group. A basic solution is needed in each case to prevent protonation of
the basic side chain to keep the amino acid electrically neutral.
The other amino acids are considered neutral, with no strongly acidic or basic side
chains. Their isoelectric points are slightly acidic (from about 5 to 6) because the
¬ NH
group is slightly more acidic than the
¬ COO
group is basic.
+
-
3
PROBLEM 24-4
Draw the structure of the predominant form of
(a) isoleucine at pH 11
(b) proline at pH 2
(c) arginine at pH 7
(d) glutamic acid at pH 7
(e) a mixture of alanine, lysine, and aspartic acid at (i) pH 6; (ii) pH 11; (iii) pH 2
Hint
problem-solving
PROBLEM 24-5
At its isoelectric point (IEP), an
Draw the resonance forms of a protonated guanidino group, and explain why arginine has
amino acid has a net charge of
such a strongly basic isoelectric point.
zero, with
NH
and
COO
+
-
3
balancing each other. In more
PROBLEM 24-6
acidic solution (lower pH), the
Although tryptophan contains a heterocyclic amine, it is considered a neutral amino acid. Ex-
carboxyl group becomes
plain why the indole nitrogen of tryptophan is more weakly basic than one of the imidazole
protonated and the net charge
nitrogens of histidine.
is positive. In more basic
solution (higher pH), the amino
Electrophoresis uses differences in isoelectric points to separate mixtures of
group loses its proton and the
amino acids (Figure 24-4). A streak of the amino acid mixture is placed in the center
net charge is negative.
of a layer of acrylamide gel or a piece of filter paper wet with a buffer solution. Two
electrodes are placed in contact with the edges of the gel or paper, and a potential of
several thousand volts is applied across the electrodes. Positively charged (cationic)
amino acids are attracted to the negative electrode (the cathode), and negatively
charged (anionic) amino acids are attracted to the positive electrode (the anode). An
amino acid at its isoelectric point has no net charge, so it does not move.
As an example, consider a mixture of alanine, lysine, and aspartic acid in a buffer
solution at pH 6. Alanine is at its isoelectric point, in its dipolar zwitterionic form with
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4 5
5 6
6 7
7 8
8 9
9 10
10 11
11 12
12 13
13 14
14 15
15 16
16 17
17 18
18 19
19 20
20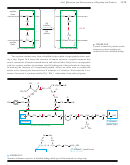 21
21 22
22 23
23 24
24 25
25 26
26 27
27 28
28 29
29 30
30 31
31 32
32 33
33 34
34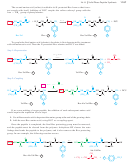 35
35 36
36 37
37 38
38 39
39 40
40 41
41 42
42 43
43 44
44 45
45 46
46 47
47