Acids And Bases Worksheet With Answers
ADVERTISEMENT
Chapter 3
Acids and Bases
Review of Concepts
Fill in the blanks below. To verify that your answers are correct, look in your textbook at
the end of Chapter 3. Each of the sentences below appears verbatim in the section
entitled Review of Concepts and Vocabulary.
• A Brønsted-Lowry acid is a proton ________, while a Brønsted-Lowry base is
a proton ________.
• The mechanism of proton transfer always involves at least _____ curved arrows.
• A strong acid has a _____ pK
, while a weak acid has a _____ pK
.
a
a
• There are four factors to consider when comparing the ___________ of conjugate
bases.
• The equilibrium of an acid-base reaction always favors the more ____________
negative charge.
• A Lewis acid is an electron ___________, while a Lewis base is an electron
____________.
Review of Skills
Fill in the blanks and empty boxes below. To verify that your answers are correct, look
in your textbook at the end of Chapter 3. The answers appear in the section entitled
SkillBuilder Review.
SkillBuilder 3.1 Drawing the Mechanism of a Proton Transfer
DRAW THE CURVED ARROWS FOR THE FOLLOWING ACID-BASE REACTION
O
+
CH
O
HO
+
CH
OH
3
3
H
H
SkillBuilder 3.2 Using pK
Values to Compare Acids
a
CIRCLE THE COMPOUND BELOW THAT IS MORE ACIDIC
O
O
H
H
O
H H
pKa = 4.75
pKa = 19.2
SkillBuilder 3.3 Using pK
Values to Compare Basicity
a
COMPARE THE FOLLOWING pK
VALUES:
AND CIRCLE THE STRONGER BASE BELOW
a
O
O
O
O
O
O
pKa = 19
pKa = 9
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4 5
5 6
6 7
7 8
8 9
9 10
10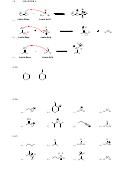 11
11 12
12 13
13 14
14 15
15 16
16 17
17








