Acids And Bases Worksheet With Answers Page 16
ADVERTISEMENT
55
CHAPTER 3
3.55.
3
a) There is only one sp
hybridized carbon atom in cyclopentadiene.
b) The most acidic proton in cyclopentadiene is highlighted below:
H
The corresponding conjugate base is highly resonance stabilized. In addition,
the conjugate base is further stabilized by yet another factor that we will
discuss in Chapter 18.
c)
3
d) There are no sp
hybridized carbon atoms in the conjugate base.
2
e) All carbon atoms are sp
hybridized and trigonal planar. Therefore, the entire
compound has planar geometry.
f) There are five hydrogen atoms in the conjugate base.
g) There is one lone pair in the conjugate base, and it is highly delocalized.
3.56. When salicylic acid is deprotonated, the resulting conjugate base is further
stabilized by intramolecular hydrogen bonding:
O
O
O
H
3.57.
O
O
or
OH
OH
a)
O
O
or
O
O
b)
(there are other possibilities as well)
OH
OH
O
or
O
c)
(there are other possibilities as well)
3.58. The four constitutional isomers are shown below.
NO
2
NO
NO
NO
2
2
2
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4 5
5 6
6 7
7 8
8 9
9 10
10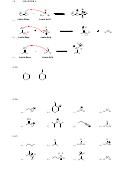 11
11 12
12 13
13 14
14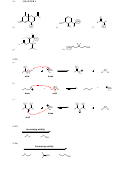 15
15 16
16 17
17








