Acids And Bases Worksheet With Answers Page 2
ADVERTISEMENT
41
CHAPTER 3
SkillBuilder 3.4 Using pK
Values to Predict the Position of Equilibrium
a
CIRCLE THE SIDE OF THE EQUILIBRIUM THAT IS FAVORED:
O
O
O
O
O
OH
H
H
pKa = 9.0
pKa = 15.7
SkillBuilder 3.5 Assessing Relative Stability. Factor #1: Atom
H
COMPARE THE TWO PROTONS SHOWN
IN THE FOLLOWING COMPOUND, AND
N
O
CIRCLE THE ONE THAT IS MORE ACIDIC.
USE THE EXTRA SPACE TO DRAW THE
H
TWO POSSIBLE CONJUGATE BASES.
SkillBuilder 3.6 Assessing Relative Stability. Factor #2: Resonance
COMPARE THE TWO PROTONS SHOWN
O
IN THE FOLLOWING COMPOUND, AND
CIRCLE THE ONE THAT IS MORE ACIDIC.
O
H
USE THE EXTRA SPACE TO DRAW THE
O
H
TWO POSSIBLE CONJUGATE BASES.
SkillBuilder 3.7 Assessing Relative Stability. Factor #3: Induction
COMPARE THE TWO PROTONS SHOWN IN
CCl
3
THE FOLLOWING COMPOUND, AND
CIRCLE THE ONE THAT IS MORE ACIDIC.
H
H
USE THE EXTRA SPACE TO DRAW THE
O
O
TWO POSSIBLE CONJUGATE BASES.
SkillBuilder 3.8 Assessing Relative Stability. Factor #4: Orbital
COMPARE THE TWO PROTONS SHOWN IN
H
H
THE FOLLOWING COMPOUND, AND
CIRCLE THE ONE THAT IS MORE ACIDIC.
USE THE EXTRA SPACE TO DRAW THE
TWO POSSIBLE CONJUGATE BASES.
SkillBuilder 3.9 Assessing Relative Stability. Using all Four Factors
COMPARE THE TWO PROTONS SHOWN IN
H
H
THE FOLLOWING COMPOUND, AND CIRCLE
THE ONE THAT IS MORE ACIDIC. USE THE
N
EXTRA SPACE TO DRAW THE TWO
POSSIBLE CONJUGATE BASES.
SkillBuilder 3.10 Predicting the Position of Equilibrium Without the Use of pK
Values
a
CIRCLE THE SIDE OF THE EQUILIBRIUM THAT IS FAVORED:
O
S
O
S
H
H
+
+
N
N
N
N
SkillBuilder 3.11 Choosing the appropriate reagent for a proton transfer reaction
DETERMINE WHETHER WATER IS A SUITABLE PROTON SOURCE TO PROTONATE THE ACETATE ION, AS SHOWN BELOW:
O
O
+
O
+
H
OH
H
H
O
O
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4 5
5 6
6 7
7 8
8 9
9 10
10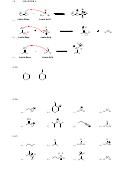 11
11 12
12 13
13 14
14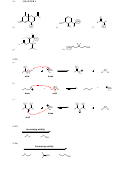 15
15 16
16 17
17








