Acids And Bases Worksheet With Answers Page 5
ADVERTISEMENT
44
CHAPTER 3
3.7.
N
O
a)
H
H
b)
O
c)
H
H
d)
O
H
H
H
C C
H
e)
f)
H
OH
3.8.
strongest base
H
N
H
H
weakest base
N
N
H
3.9.
a) A lone pair on a nitrogen atom will be more basic than a lone pair on an oxygen atom.
b) The lone pair on the nitrogen atom is thirteen orders of magnitude more basic than the
lone pair on the oxygen atom.
3.10.
a) left side
b) right side
c) right side
d) right side
3.11. The equilibrium does not favor deprotonation of acetylene by hydroxide, because
water is more acidic than acetylene. The equilibrium will favor the weaker acid
(acetylene). A suitable base would be one whose conjugate acid is less acidic than
acetylene. For example, H
N would be a suitable base, because ammonia (NH
) is less
2
3
acidic than acetylene.
O
H H
N
O
H
glycine
3.12.
3.13.
H
H
H C N
H
H
O
H
a)
b)
O
N H
HS
H
OH
c)
d)
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4 5
5 6
6 7
7 8
8 9
9 10
10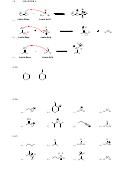 11
11 12
12 13
13 14
14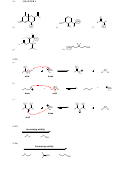 15
15 16
16 17
17








