Chemistry Worksheet With Answer Key - Chem 10113, Quiz 1b Page 20
ADVERTISEMENT
angle = 120
=> Larger bond angle
angle = <109.5
5. (5 points) When a thin glass tube is put into water, the water rises 1.4 cm. When the
same tube is put into hexane, the hexane rises only 0.4 cm. Explain the difference.
Water can generate strong adhesive interactions with the glass (due to the
dipoles at the surface of the glass), but hexane is nonpolar and cannot interact
strongly with the glass surface.
6. (12 points) For the following organic molecule, give the valence bond description of
the bonding. For the sketch, show only one example of each type of bond. (e.g. in H
O
2
only draw one O-H bond)
Is bond or ?
Formula
EG at
Hybridization
Draw the orbital
central
at central
interaction(s)
atom
atom
3
SBr
4
sp
2
3
2
IF
6
sp
d
5
7. (12 points) a) Label the strongest type of intermolecular attraction for each material,
then b) explain the melting point data in terms of molecular attractive forces.
a)
20
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1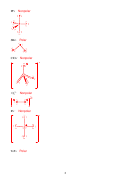 2
2 3
3 4
4 5
5 6
6 7
7 8
8 9
9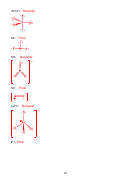 10
10 11
11 12
12 13
13 14
14 15
15 16
16 17
17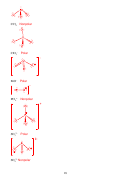 18
18 19
19 20
20 21
21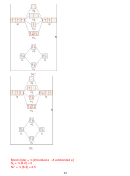 22
22 23
23 24
24








