Chemistry Worksheet With Answer Key - Chem 10113, Quiz 1b Page 15
ADVERTISEMENT
-
-
Bond Order = ½ [# bonded e
- # antibonded e
]
C
= ½ [6-2] = 2
2
+
C
= ½ [5-2] = 1.5
2
-
C
= ½ [7-2] = 2.5
2
+
-
Bond Length: C
> C
> C
2
2
2
-
+
Bond Energy: C
> C
> C
2
2
2
9. (6 points) In each blank write >, < or = as appropriate.
Strength of intermolecular
=
Strength of intramolecular
forces in MgCl
forces in MgCl
2
2
Viscosity of CH
OH
>
Viscosity of H
CO
3
2
bp of CS
>
bp of CO
2
2
10. (12 points) Briefly define the following terms.
equatorial (in VSEPR theory) –
In the tbp shape, the three terminal positions that
are coplanar and at 120 angles from each other. (labeled e in the pic)
15
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1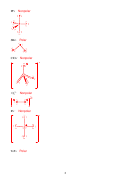 2
2 3
3 4
4 5
5 6
6 7
7 8
8 9
9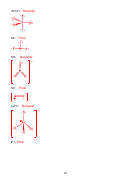 10
10 11
11 12
12 13
13 14
14 15
15 16
16 17
17 18
18 19
19 20
20 21
21 22
22 23
23 24
24








