Chemistry Worksheet With Answer Key - Chem 10113, Quiz 1b Page 21
ADVERTISEMENT
mp
Strongest intermolecular attractive force
London Dispersion Force
SiH
-185 °C
4
London Dispersion Force
Kr
-157 °C
HBr
-87 °C
Dipole-dipole
NCl
-40 °C
Dipole-dipole
3
b) Explain:
dipole-dipole > London Dispersion Force
The dipole-dipole in NCl
is greater than that of HBr due to NCl
being larger than
3
3
HBr, which is why it has the higher mp. Also, the LDF in Kr is greater than that of
SiH
due to Kr being larger than SiH
, which is why it has the higher mp.
4
4
mp increases with stronger forces because it takes more energy to break apart
strong forces.
8. (14 points) SHOW ALL WORK. According to MO theory, which has the highest
−
+
bond order, bond energy, and shortest bond length? N
, N
, or N
? Include an MO
2
2
2
diagram in your answer.
21
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1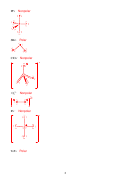 2
2 3
3 4
4 5
5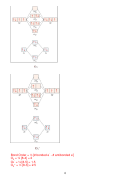 6
6 7
7 8
8 9
9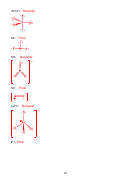 10
10 11
11 12
12 13
13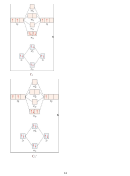 14
14 15
15 16
16 17
17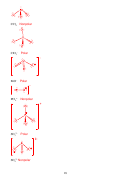 18
18 19
19 20
20 21
21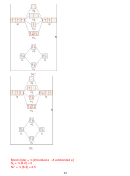 22
22 23
23 24
24








