Chemistry Worksheet With Answer Key - Chem 10113, Quiz 1b Page 7
ADVERTISEMENT
-
+
Bond Length: O
> O
> O
2
2
2
+
-
Bond Energy: O
> O
> O
2
2
2
9. (6 points) In each blank write >, < or = as appropriate.
Strength of intermolecular
<
Strength of intramolecular
forces in H
O
forces in CH
OH
2
3
Viscosity of CH
<
Viscosity of CH
Cl
4
3
bp of CO
<
bp of NO
2
2
10. (12 points) Briefly define the following terms.
axial (in VSEPR theory) –
Location of atoms that are above & below the equatorial
plane in a trigonal bipyramidal structure (labeled as a in pic)
a
e
e
e
a
bond –
head-to-head overlap along the bond axis with zero nodes along the
bonding axis
triple point –
T and P where all 3 phases coexist in equilibrium
11. (18 points) SHOW ALL WORK. The vapor pressure of CCl
F at 300 K is 856 torr.
3
If 12.6 g of CCl
F is enclosed in a 1.0-L container, will any liquid be present? If so, what
3
mass of liquid?
856 torr x 1 atm = 1.1263 atm
760 torr
n = PV = 1.12632 atm x 1.0 L = 0.04575 mol
RT
0.008206 Latm x 300 K
Kmol
0.04575 mol x 137.36 g = 6.2845 g
1 mol
12.6 g – 6.2845 = 6.3 g
7
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1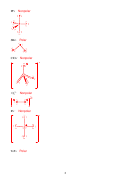 2
2 3
3 4
4 5
5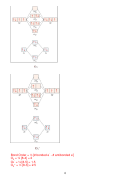 6
6 7
7 8
8 9
9 10
10 11
11 12
12 13
13 14
14 15
15 16
16 17
17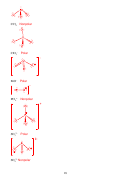 18
18 19
19 20
20 21
21 22
22 23
23 24
24








