Clil Module Periodic Table Of The Elements Worksheet - Prof.ssa Elena Tibaldi - Istituto Sant''Anna - 2012-2013 Page 20
ADVERTISEMENT
PERIODIC PROPERTIES OF THE ELEMENTS
• In the periodic table, elements are placed in order of increasing atomic number.
• The period number corresponds to the number of shells occupied by the electrons.
• The group number corresponds to the number of electrons in the outermost shell.
• The electrons of the outer level are involved in intramolecular bonding formation.
• Elements in the same group (column of the periodic table) have similar properties.
MELTING AND BOILING POINTS
depends on the structure of the element and on
the type of attractive forces holding the atoms together. It decreases down a group.
ATOMIC RADIUS
decreases across the period (Ex. F<Li); increases down a group (ex.
K>Li). It is the distance from the nucleus to the outermost electron or the half distance
between the nuclei of two bounded atoms of the same element.
ELECTRONEGATIVITY
increases across the period (Ex. F>Li) and decreases down a
group (ex. K<Li). It represents the relative measure of the attraction that an atom has for
a shared pair of electrons when it is covalently bounded to another atom. The most
electronegative
elements
are,
in
order,
F>O>Cl=N>Br>C.
Non-metals
are
more
electronegative than metals.
20
Prof.ssa Elena Tibaldi - Istituto Sant’Anna – AS 2012-13
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4 5
5 6
6 7
7 8
8 9
9 10
10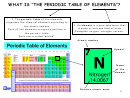 11
11 12
12 13
13 14
14 15
15 16
16 17
17 18
18 19
19 20
20 21
21 22
22 23
23 24
24 25
25 26
26 27
27 28
28 29
29 30
30 31
31 32
32 33
33 34
34 35
35 36
36 37
37 38
38 39
39 40
40 41
41 42
42 43
43 44
44 45
45 46
46 47
47 48
48








