Clil Module Periodic Table Of The Elements Worksheet - Prof.ssa Elena Tibaldi - Istituto Sant''Anna - 2012-2013 Page 9
ADVERTISEMENT
REVIEW: THE STRUCTURE OF THE ATOM
Atomic particle that
Nuclear particle that
has a negative charge
has a positive charge
Electrons are smaller
in size than protons.
In an electrically neutral
atom, the number of protons
(atomic number) equals the
number of electrons.
Valency is the
number of electrons
If an atom gains electron
that can be gained or
becomes a negative ion; if
lost by an atom.
an atom loses electrons,
becomes a positive ion.
Isotopes are atoms of the
same element that have the
Nuclear particle that
same atomic number, but
has no charge
different mass number,
because of the different
Part of the atom
The mass number is the
number of neutrons in the
containing protons and
sum of protons and
nucleus
neutrons
9
neutrons in the nucleus.
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3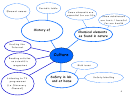 4
4 5
5 6
6 7
7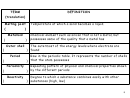 8
8 9
9 10
10 11
11 12
12 13
13 14
14 15
15 16
16 17
17 18
18 19
19 20
20 21
21 22
22 23
23 24
24 25
25 26
26 27
27 28
28 29
29 30
30 31
31 32
32 33
33 34
34 35
35 36
36 37
37 38
38 39
39 40
40 41
41 42
42 43
43 44
44 45
45 46
46 47
47 48
48








