Clil Module Periodic Table Of The Elements Worksheet - Prof.ssa Elena Tibaldi - Istituto Sant''Anna - 2012-2013 Page 34
ADVERTISEMENT
GROUP 7 ELEMENTS: THE HALOGENS
Halogens are present in nature as diatomic molecules, made of two atoms linked together
with one covalent bond.
They have seven electrons in the outer shell, therefore they tend to gain one electron to
complete the outer shell and become negative ions (ex. F
, Cl
).
-
-
They have a very low melting and boiling point.
Reactivity decreases down the group
chlorine (Cl
)is more reactive than bromine (Br
).
2
2
Chlorine
Fluorine
• present as diatomic molecule (Cl
):
2
• present as diatomic molecule (F
)
• strong-smelling poisonous green gas
2
• poisonous yellow gas
• added to water as disinfectant (ie in
• most reactive element known
swimming pools)
• used in treatment of water
• used in toothpaste
• main component of teflon in nonstick pans
34
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4 5
5 6
6 7
7 8
8 9
9 10
10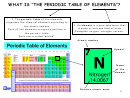 11
11 12
12 13
13 14
14 15
15 16
16 17
17 18
18 19
19 20
20 21
21 22
22 23
23 24
24 25
25 26
26 27
27 28
28 29
29 30
30 31
31 32
32 33
33 34
34 35
35 36
36 37
37 38
38 39
39 40
40 41
41 42
42 43
43 44
44 45
45 46
46 47
47 48
48








