Clil Module Periodic Table Of The Elements Worksheet - Prof.ssa Elena Tibaldi - Istituto Sant''Anna - 2012-2013 Page 23
ADVERTISEMENT
ELECTRONIC CONFIGURATION OF THE ELEMENTS
Sub Shells and Orbitals
Sub shells are known by letters s, p, d, and f.
Electrons occupy negative charge clouds called
orbitals, each orbital can hold only 2 electrons with
opposite spin.
Each type of shell has a different type of orbital.
s orbital
max 2 electrons
p orbital
max 6 electrons
d orbital
max 10 electrons
f orbital
max 14 electrons
Orbital must be filled in the following order:
1s 2s 2p 3s 3p 4s 3d 4p 5s 4d 5p......
Example: Carbon (C) has 6 electrons:
23
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3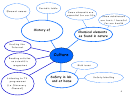 4
4 5
5 6
6 7
7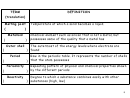 8
8 9
9 10
10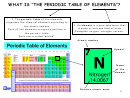 11
11 12
12 13
13 14
14 15
15 16
16 17
17 18
18 19
19 20
20 21
21 22
22 23
23 24
24 25
25 26
26 27
27 28
28 29
29 30
30 31
31 32
32 33
33 34
34 35
35 36
36 37
37 38
38 39
39 40
40 41
41 42
42 43
43 44
44 45
45 46
46 47
47 48
48








