Clil Module Periodic Table Of The Elements Worksheet - Prof.ssa Elena Tibaldi - Istituto Sant''Anna - 2012-2013 Page 37
ADVERTISEMENT
THE TRANSITION ELEMENTS
One group of transition elements is found between group II and III.
Transition elements have similar physical properties:
• have higher densities than group I and group II metals
• have higher melting points and boiling points than group I and group II metals because
they have strong inter-atomic forces
• are harder and stronger than group I and group II metals
• are good conductors of electricity and heat
Transition elements have similar chemical properties:
• are less reactive than group I and group II metals because it is more difficult to lose
electrons
• do not react with water but can form oxides when reacted with steam
• they can have more that one valancy. Example: iron can lose two electrons (
Fe
) or
2+
three electrons (
Fe
)
3+
• form coloured compounds and solutions
Some of transition elements are commonly used as follows:
• copper (Cu)
in alloys with tin (Sn) (
bronze) and with zinc (Zn) (
brass)
• iron (Fe)
highly reactive with oxygen (
rust); used in alloys with carbon (C) (
steel)
• lead (Pb)
metal elements whose compounds are toxic; used for containers that protect
against harmful radiation; poor electricity conductor; does not corrode easily
• titanium (Ti)
aircraft, hip joint replacement
37
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4 5
5 6
6 7
7 8
8 9
9 10
10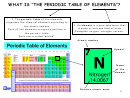 11
11 12
12 13
13 14
14 15
15 16
16 17
17 18
18 19
19 20
20 21
21 22
22 23
23 24
24 25
25 26
26 27
27 28
28 29
29 30
30 31
31 32
32 33
33 34
34 35
35 36
36 37
37 38
38 39
39 40
40 41
41 42
42 43
43 44
44 45
45 46
46 47
47 48
48








