Clil Module Periodic Table Of The Elements Worksheet - Prof.ssa Elena Tibaldi - Istituto Sant''Anna - 2012-2013 Page 24
ADVERTISEMENT
GROUP 1 ELEMENTS: ALKALI METALS
Group 1 elements are called alkali metals. The most common alkali metals are: lithium,
sodium and potassium.
All of the alkali metals have similar physical properties:
• are soft metals
• are shiny and silvery when cut
• are good conductors of electricity and heat
• have low melting points and boiling points; melting points and boiling points decrease
down the group
• have low densities and the density generally increases down the group
All of the alkali metals have similar chemical properties:
• are very reactive with water and oxygen, therefore they must be stored in oil
• they tend to lose one electron from the outer shell and become positive ions (ex. Na
)
+
• reactivity increases down the group (Ex. K>Li) as the outer shell electron is further
away from the nucleus.
• they react with oxygen forming oxides (ex. K
O potassium oxide)
2
• they react with water to produce an acqueous solution of hydroxide and hydrogen (ex.
2Na + 2H
O
2NaOH + H
)
2
2
Alkali metals compounds:
• are very stable and difficult to separate by chemical means
• do not decompose on heating and are soluble in water
24
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3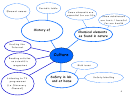 4
4 5
5 6
6 7
7 8
8 9
9 10
10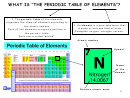 11
11 12
12 13
13 14
14 15
15 16
16 17
17 18
18 19
19 20
20 21
21 22
22 23
23 24
24 25
25 26
26 27
27 28
28 29
29 30
30 31
31 32
32 33
33 34
34 35
35 36
36 37
37 38
38 39
39 40
40 41
41 42
42 43
43 44
44 45
45 46
46 47
47 48
48








