Chemistry Annual Curriculum Map
ADVERTISEMENT
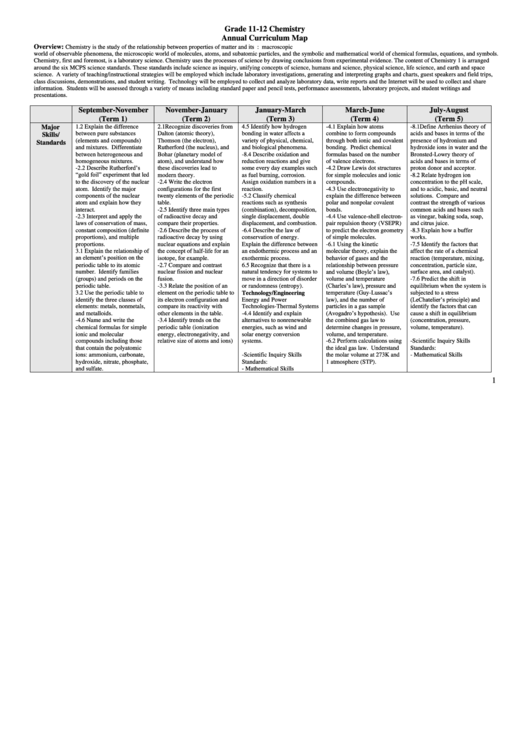
Grade 11-12 Chemistry
Annual Curriculum Map
Overview:
Chemistry is the study of the relationship between properties of matter and its structure. Chemistry requires students to move among three domains of thought: macroscopic
world of observable phenomena, the microscopic world of molecules, atoms, and subatomic particles, and the symbolic and mathematical world of chemical formulas, equations, and symbols.
Chemistry, first and foremost, is a laboratory science. Chemistry uses the processes of science by drawing conclusions from experimental evidence. The content of Chemistry 1 is arranged
around the six MCPS science standards. These standards include science as inquiry, unifying concepts of science, humans and science, physical science, life science, and earth and space
science. A variety of teaching/instructional strategies will be employed which include laboratory investigations, generating and interpreting graphs and charts, guest speakers and field trips,
class discussions, demonstrations, and student writing. Technology will be employed to collect and analyze laboratory data, write reports and the Internet will be used to collect and share
information. Students will be assessed through a variety of means including standard paper and pencil tests, performance assessments, laboratory projects, and student writings and
presentations.
September-November
November-January
January-March
March-June
July-August
(Term 1)
(Term 2)
(Term 3)
(Term 4)
(Term 5)
1.2 Explain the difference
2.1Recognize discoveries from
4.5 Identify how hydrogen
-4.1 Explain how atoms
-8.1Define Arrhenius theory of
Major
between pure substances
Dalton (atomic theory),
bonding in water affects a
combine to form compounds
acids and bases in terms of the
Skills/
(elements and compounds)
Thomson (the electron),
variety of physical, chemical,
through both ionic and covalent
presence of hydronium and
Standards
and mixtures. Differentiate
Rutherford (the nucleus), and
and biological phenomena.
bonding. Predict chemical
hydroxide ions in water and the
between heterogeneous and
Bohar (planetary model of
-8.4 Describe oxidation and
formulas based on the number
Bronsted-Lowry theory of
homogeneous mixtures.
atom), and understand how
reduction reactions and give
of valence electrons.
acids and bases in terms of
-2.2 Describe Rutherford’s
these discoveries lead to
some every day examples such
-4.2 Draw Lewis dot structures
proton donor and acceptor.
“gold foil” experiment that led
modern theory.
as fuel burning, corrosion.
for simple molecules and ionic
-8.2 Relate hydrogen ion
to the discovery of the nuclear
-2.4 Write the electron
Assign oxidation numbers in a
compounds.
concentration to the pH scale,
atom. Identify the major
configurations for the first
reaction.
-4.3 Use electronegativity to
and to acidic, basic, and neutral
components of the nuclear
twenty elements of the periodic
-5.2 Classify chemical
explain the difference between
solutions. Compare and
atom and explain how they
table.
reactions such as synthesis
polar and nonpolar covalent
contrast the strength of various
interact.
-2.5 Identify three main types
(combination), decomposition,
bonds.
common acids and bases such
-2.3 Interpret and apply the
of radioactive decay and
single displacement, double
-4.4 Use valence-shell electron-
as vinegar, baking soda, soap,
laws of conservation of mass,
compare their properties.
displacement, and combustion.
pair repulsion theory (VSEPR)
and citrus juice.
constant composition (definite
-2.6 Describe the process of
-6.4 Describe the law of
to predict the electron geometry
-8.3 Explain how a buffer
proportions), and multiple
radioactive decay by using
conservation of energy.
of simple molecules.
works.
proportions.
nuclear equations and explain
Explain the difference between
-6.1 Using the kinetic
-7.5 Identify the factors that
3.1 Explain the relationship of
the concept of half-life for an
an endothermic process and an
molecular theory, explain the
affect the rate of a chemical
an element’s position on the
isotope, for example.
exothermic process.
behavior of gases and the
reaction (temperature, mixing,
periodic table to its atomic
-2.7 Compare and contrast
6.5 Recognize that there is a
relationship between pressure
concentration, particle size,
and volume (Boyle’s law),
number. Identify families
nuclear fission and nuclear
natural tendency for systems to
surface area, and catalyst).
(groups) and periods on the
fusion.
move in a direction of disorder
volume and temperature
-7.6 Predict the shift in
(Charles’s law), pressure and
periodic table.
-3.3 Relate the position of an
or randomness (entropy).
equilibrium when the system is
3.2 Use the periodic table to
element on the periodic table to
temperature (Guy-Lussac’s
subjected to a stress
Technology/Engineering
(LeChatelier’s principle) and
identify the three classes of
its electron configuration and
Energy and Power
law), and the number of
elements: metals, nonmetals,
compare its reactivity with
Technologies-Thermal Systems
particles in a gas sample
identify the factors that can
(Avogadro’s hypothesis). Use
and metalloids.
other elements in the table.
-4.4 Identify and explain
cause a shift in equilibrium
-4.6 Name and write the
-3.4 Identify trends on the
alternatives to nonrenewable
the combined gas law to
(concentration, pressure,
chemical formulas for simple
periodic table (ionization
energies, such as wind and
determine changes in pressure,
volume, temperature).
ionic and molecular
energy, electronegativity, and
solar energy conversion
volume, and temperature.
compounds including those
relative size of atoms and ions)
systems.
-6.2 Perform calculations using
-Scientific Inquiry Skills
that contain the polyatomic
the ideal gas law. Understand
Standards:
ions: ammonium, carbonate,
-Scientific Inquiry Skills
the molar volume at 273K and
- Mathematical Skills
hydroxide, nitrate, phosphate,
Standards:
1 atmosphere (STP).
and sulfate.
- Mathematical Skills
1
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Business
 1
1 2
2 3
3