Bonding Concepts Worksheet - Unit 8/9, Michalek Page 6
ADVERTISEMENT
AP Chemistry - Michalek
6
Unit 08 Bonding Concepts
Worksheet 8.01
Name______________________________
Lattice Energy
1. Circle the compound in the pair of ionic substances that has the most exothermic lattice energy.
Sodium Chloride / Potassium Chloride
Lithium Fluoride / Lithium Chloride
Magnesium Hydroxide / Magnesium Oxide
Iron (III) Hydroxide / Iron (II) Hydroxide
2. Use the following data to estimate ΔH
for sodium chloride. Na (s) + ½ Cl
→ NaCl.
f
2
Lattice Energy
-786 kJ/mol
Ionization Energy for Na
495 kJ/mol
Electron Affinity of Cl
-349 kJ/mol
Bond Energy of Cl
239 kJ/mol
2
Enthalpy of sublimation of Na
109 kJ/mol
3. Why are some bonds ionic and some covalent?
4. Rank the following in order of increasing ionic character: N—O, Ca—O, C—F, Br—Br, K—F.
5. Give three ions that are isoelectronic with neon. Place them in order of increasing ionic size.
6. Give three ions that are isoelectronic with Argon. Place them in order of decreasing ionization energy.
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4 5
5 6
6 7
7 8
8 9
9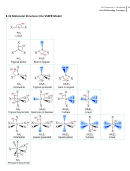 10
10 11
11 12
12 13
13 14
14 15
15 16
16 17
17 18
18 19
19 20
20 21
21 22
22 23
23 24
24 25
25 26
26 27
27 28
28 29
29 30
30








