Chem 1101 2014-J-3 Worksheet With Answers - The University Of Sydney - 2014 Page 10
ADVERTISEMENT
CHEM1101
2010-N-5
November 2010
Marks
• Complete the table below showing the number of valence electrons, the Lewis
6
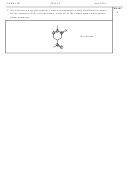
structures and the predicted shapes of the following species. Ammonia, NH
, is
3
given as an example.
Number of electron
Formula
pairs on central
Lewis Structure
Name of
atom (discounting
molecular shape
multiple bonds)
H
N
H
NH
4
trigonal pyramidal
3
H
ClF
5
T-shaped
3
3–
PO
4
tetrahedral
4
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4 5
5 6
6 7
7 8
8 9
9 10
10 11
11 12
12 13
13 14
14 15
15 16
16 17
17 18
18 19
19 20
20 21
21








