Chem 1101 2014-J-3 Worksheet With Answers - The University Of Sydney - 2014 Page 17
ADVERTISEMENT
CHEM1101
2006-N-6
November 2006
Marks
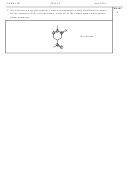
• Complete the table below showing the number of valence electrons, the Lewis
5
structure and the predicted shape of each of the following species.
Total number of
Formula
Lewis structure
Geometry of species
valence electrons
H
N
H
e.g. NH
8
trigonal pyramidal
3
H
O
–
NO
24
trigonal planar
3
N
O
O
HCN
10
linear
H
C
N
–
Which of NH
, NO
and HCN have a non-zero dipole moment?
3
3
-
NH
and HCN both have dipole moments. Although the N-O bonds in NO
are
3
3
polar, the trigonal planar shape means that these cancel and there is no overall
dipole moment.
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4 5
5 6
6 7
7 8
8 9
9 10
10 11
11 12
12 13
13 14
14 15
15 16
16 17
17 18
18 19
19 20
20 21
21








