Chem 1101 2014-J-3 Worksheet With Answers - The University Of Sydney - 2014 Page 7
ADVERTISEMENT
CHEM1101
2012-J-3
June 2012
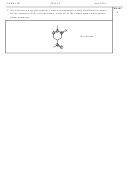
• The σ-bonding in two plausible structures of ozone, O
, is shown below. Complete
3
4
each structure by adding electrons and/or π-bonds as appropriate.
Predict the geometry of ozone? Give reasons for your answer.
Ozone adopts the non-cyclic structure. The cyclic structure is very strained with
bond angles of 60° instead of 109.5°, making it very unstable. In contrast, the
second structure is stabilised by resonance.
Ozone does not contain 1 double and 1 single bond. Both the O-O bonds are
exactly the same length and true structure is a sort of average of the two Lewis
structures shown. The energy of the true structure is lower than the theoretical
energy for either of the given structures. This energy difference is known as
resonance stabilisation energy.
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4 5
5 6
6 7
7 8
8 9
9 10
10 11
11 12
12 13
13 14
14 15
15 16
16 17
17 18
18 19
19 20
20 21
21








