Chem 1101 2014-J-3 Worksheet With Answers - The University Of Sydney - 2014 Page 9
ADVERTISEMENT
CHEM1101
2012-N-6
November 2012
Marks
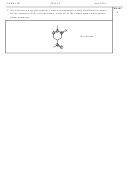
g) In the atmosphere, nitrogen oxides exist in many forms, including NO and NO
. Two
2
6
other forms are N
O and N
O
(the dimer of NO
). Draw Lewis structures for both
2
2
4
2
N
O and N
O
. Examine your structures closely. If you can draw a second, valid,
2
2
4
Lewis structure, draw it underneath.
N
O structure
N
O
structure
2
2
4
Second structure, if appropriate
Second structure, if appropriate
4
h) Use VSEPR theory to determine the shape of N
O and N
O
. Sketch the shape below
2
2
4
and indicate the approximate bond angle for all angles in the molecule. Be clear in
your sketch as to planar and non-planar structures where appropriate. Hence, or
otherwise, indicate whether either molecule has a permanent dipole moment.
N
O
N
O
2
2
4
o
~120
o
180
O
O
o
N
N
~120
N
N
O
O
O
Dipole moment? YES / NO
Dipole moment? YES / NO
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4 5
5 6
6 7
7 8
8 9
9 10
10 11
11 12
12 13
13 14
14 15
15 16
16 17
17 18
18 19
19 20
20 21
21








