Chem 1101 2014-J-3 Worksheet With Answers - The University Of Sydney - 2014 Page 11
ADVERTISEMENT
CHEM1101
2009-J-8
June 2009
Marks
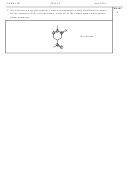
Draw the major resonance contributors of nitryl chloride, ClNO
.
2
2
O
O
Cl
N
Cl
N
O
O
The bond order is an average over
What is the bond order of the N–O bonds?
the resonance structures: 1.5
Complete the following table showing the number of valence electrons, a Lewis
5
structure and the predicted shape of each of the following species.
Number of
Molecule
Chemical
valence
Lewis structure
Geometry of species
name
formula
electrons
O
e.g. water
H
O
8
bent
2
H
H
_
2
O
2–
carbonate ion
CO
24
trigonal planar
3
O C
O
F
chlorine
ClF
28
T-shaped
F
Cl
3
trifluoride
F
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4 5
5 6
6 7
7 8
8 9
9 10
10 11
11 12
12 13
13 14
14 15
15 16
16 17
17 18
18 19
19 20
20 21
21








