Chem 1101 2014-J-3 Worksheet With Answers - The University Of Sydney - 2014 Page 5
ADVERTISEMENT
CHEM1101
2013-J-7
June 2013
Marks
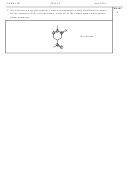
• Complete the following table for the molecules SF
and SF
.
6
4
6
Molecule Total number of
Lewis structure
Shape of molecule
valence electrons
SF
48
octahedral
6
SF
34
“see saw”
4
Sulfur hexafluoride (SF
) is quite inert, whilst sulfur tetrafluoride (SF
) is highly
6
4
reactive. Suggest a reason for the difference in reactivity between SF
and SF
.
6
4
There is a lone pair of electrons on the S that can participate in reactions for
SF
, but not for SF
.
4
6
ADVERTISEMENT
0 votes
Related Articles
Related forms
Related Categories
Parent category: Education
 1
1 2
2 3
3 4
4 5
5 6
6 7
7 8
8 9
9 10
10 11
11 12
12 13
13 14
14 15
15 16
16 17
17 18
18 19
19 20
20 21
21








